Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It . A buffer solution is used to. What is a buffer solution? A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. A buffer solution is a solution which resists changes in ph when small amounts of acids or alkalis are added. To know how to use the henderson. Buffers allow chemists to maintain a specific ph range for a reaction. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson.
from www.slideserve.com
A buffer solution is used to. A buffer solution is a solution which resists changes in ph when small amounts of acids or alkalis are added. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. To know how to use the henderson. An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. Buffers allow chemists to maintain a specific ph range for a reaction. What is a buffer solution?
PPT Buffers PowerPoint Presentation, free download ID5687114
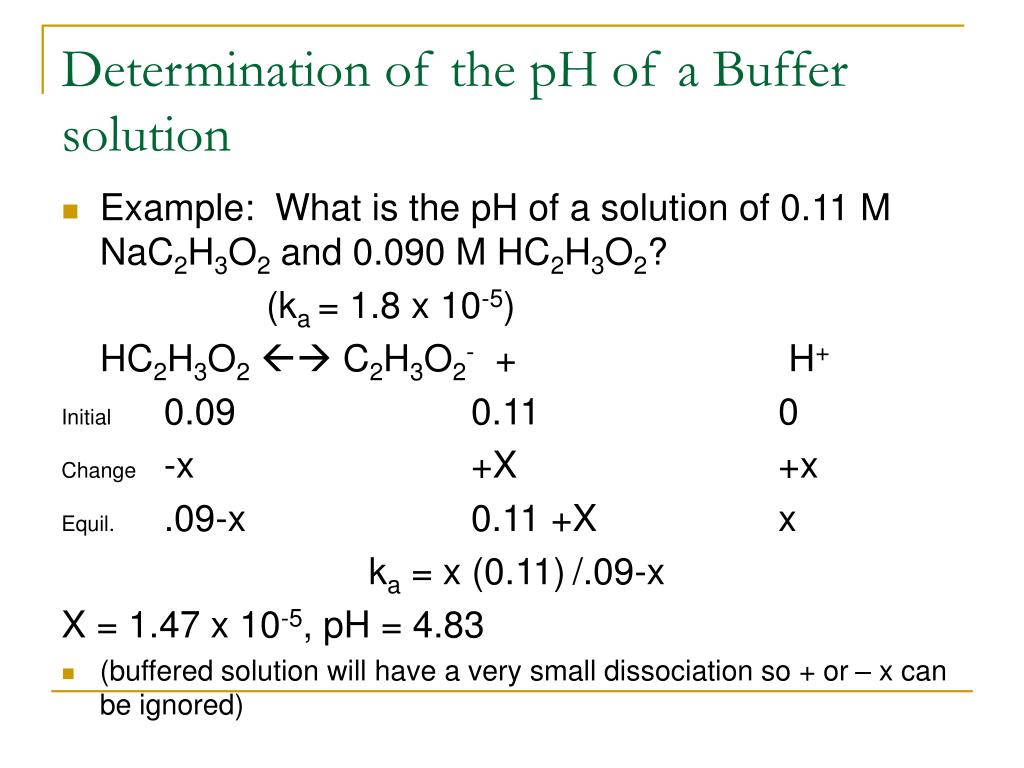
Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It Buffers allow chemists to maintain a specific ph range for a reaction. What is a buffer solution? An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. To know how to use the henderson. Buffers allow chemists to maintain a specific ph range for a reaction. A buffer solution is a solution which resists changes in ph when small amounts of acids or alkalis are added. A buffer solution is used to. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It Buffers allow chemists to maintain a specific ph range for a reaction. What is a buffer solution? Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. A buffer solution is used to. A buffer solution is a solution which resists changes in ph when small amounts of acids or alkalis. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From chemguru.sg
Calculate pH of Buffer Solution Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It A buffer solution is a solution which resists changes in ph when small amounts of acids or alkalis are added. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffer solution is used to. What is a buffer solution? Buffers allow chemists to maintain a. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.youtube.com
Buffer solution and Buffer action part 2 YouTube Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It To know how to use the henderson. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. What is a buffer solution? A buffer solution is used to. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson.. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.slideserve.com
PPT Mechanism of buffer action and buffer preparation PowerPoint Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. A buffer solution is a solution which resists changes in ph when small. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It What is a buffer solution? A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. To know how to use the henderson. A buffer solution is used to.. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From courses.lumenlearning.com
Buffers Chemistry Atoms First Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. What is a buffer solution? Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. Buffers allow chemists to maintain a specific ph range for a reaction. A. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.youtube.com
HOW ACIDIC BUFFER SOLUTION MAINTAIN ITS pH YouTube Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. Buffers allow chemists to maintain a specific ph range for a reaction. A buffer solution is used to. Calculate the ph of a buffer before and after the addition of added acid. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.youtube.com
Buffer Action Acidic and basic buffer Action how buffer resist pH Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. Buffers allow chemists to maintain a specific ph range for a reaction. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It Buffers allow chemists to maintain a specific ph range for a reaction. A buffer solution is used to. What is a buffer solution? A buffer solution is a solution which resists changes in ph when small amounts of acids or alkalis are added. A buffer solution is one which resists changes in ph when small quantities of an acid or. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From slideplayer.com
BUFFER SOLUTIONS. ppt download Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It To know how to use the henderson. Buffers allow chemists to maintain a specific ph range for a reaction. An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. A buffer solution is one which resists changes in ph when small quantities. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It To know how to use the henderson. What is a buffer solution? A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffer solution is a solution which resists changes in ph when small amounts of acids or alkalis are added. An acidic buffer is made. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. What is a buffer solution? A buffer solution is. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. To know how to use the henderson. Buffers allow chemists to maintain a. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.pinterest.com
Understanding Buffer Solution Chemistry A Complete Guide Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. Buffers allow chemists to maintain a specific ph range for a reaction. A buffer solution is used to. To know how to use the henderson. A buffer solution is one which resists. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. To know how to use the henderson. A buffer solution is a solution which resists changes in ph. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.youtube.com
Buffer Solutions Henderson Equation Acidic and Basic Buffer Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It A buffer solution is used to. Buffers allow chemists to maintain a specific ph range for a reaction. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. To know how to use the henderson. An acidic buffer is made by adding a weak acid to a solution of one of. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From riaquueta45.blogspot.com
What Is Meant By Buffer Solution Give An Example Of Basic Buffer Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. What is a buffer solution? To know how to use the henderson. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It A buffer solution is used to. What is a buffer solution? Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. Buffers allow chemists to maintain a specific ph range for a reaction. An acidic buffer is made by adding a weak acid to a solution of one of its salts. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.doubtnut.com
What are buffer solutions and how are they prepared? Explain the buffe Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It Buffers allow chemists to maintain a specific ph range for a reaction. To know how to use the henderson. A buffer solution is used to. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. What is a buffer solution? A buffer solution is one which resists changes in ph when. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.youtube.com
pH of buffer solutions the HendersonHasselbalch equation. YouTube Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. A buffer solution is used to. A buffer solution is one which resists. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. To know how to use the henderson. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. A buffer solution is a solution which resists changes in ph. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.slideserve.com
PPT Buffer This PowerPoint Presentation, free download ID2841454 Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It A buffer solution is a solution which resists changes in ph when small amounts of acids or alkalis are added. Buffers allow chemists to maintain a specific ph range for a reaction. To know how to use the henderson. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. An acidic. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From id.pinterest.com
buffer solution , Acidic & basic buffer,weak acid +salt of weak acid Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It A buffer solution is a solution which resists changes in ph when small amounts of acids or alkalis are added. An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. What is a buffer solution? Calculate the ph of a buffer before. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From byjus.com
For preparing a buffer of pH 6 by mixing sodium acetate and acetic acid Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It Buffers allow chemists to maintain a specific ph range for a reaction. An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. What is a buffer solution? To know how to use the henderson. Calculate the ph of a buffer before and. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. To know how to use the henderson. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. What is a buffer solution? A buffer solution is a solution. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. To know how to use the henderson. An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. A buffer solution is a solution. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From slidetodoc.com
Mechanism of buffer action and buffer preparation Maintaining Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It What is a buffer solution? Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. A buffer solution is a solution which resists changes in ph when small amounts of acids or alkalis are added. To know how to use the henderson. Buffers allow chemists to maintain a specific ph range. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. A buffer solution is used to. A buffer solution is a solution which resists changes in ph when small amounts of acids or alkalis are added. What is a buffer solution? Buffers. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It A buffer solution is used to. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. To know how to use the henderson. Buffers allow chemists to maintain a specific ph range for a reaction. An acidic buffer is made by adding a weak acid to a solution of one of. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffer solution is a solution which resists changes in ph when small amounts of acids or alkalis are added. A buffer solution is used to. Buffers allow chemists to maintain a specific ph range for a. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.youtube.com
Buffer Solution Buffer Action Types of buffer solution acidic and Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. What is a buffer solution? To know how to. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.youtube.com
Mechanism of Acidic Buffer and Basic Buffer Solution Chemical Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Buffers allow chemists to maintain a specific ph range for a reaction. An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From chemistrypubs.com
Buffer Solution Definition, Examples, Mechanisms, Preparations Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It A buffer solution is used to. A buffer solution is a solution which resists changes in ph when small amounts of acids or alkalis are added. Calculate the ph of a buffer before and after the addition of added acid or base using the henderson. What is a buffer solution? Buffers allow chemists to maintain a specific ph range for. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From www.youtube.com
The buffering action of an acidic buffer is maximum when its pH equals Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It A buffer solution is used to. What is a buffer solution? A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. To know how to use the henderson. A buffer solution is a solution which resists changes in ph when small amounts of acids or alkalis are. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.
From general.chemistrysteps.com
pH of a Buffer Solution Chemistry Steps Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It What is a buffer solution? Buffers allow chemists to maintain a specific ph range for a reaction. To know how to use the henderson. A buffer solution is used to. An acidic buffer is made by adding a weak acid to a solution of one of its salts (its conjugate base) like for example, ethanoic acid and sodium. A buffer. Explain Buffer Action Of Acidic Buffer Solution And Evaluate Equation Of Ph For It.