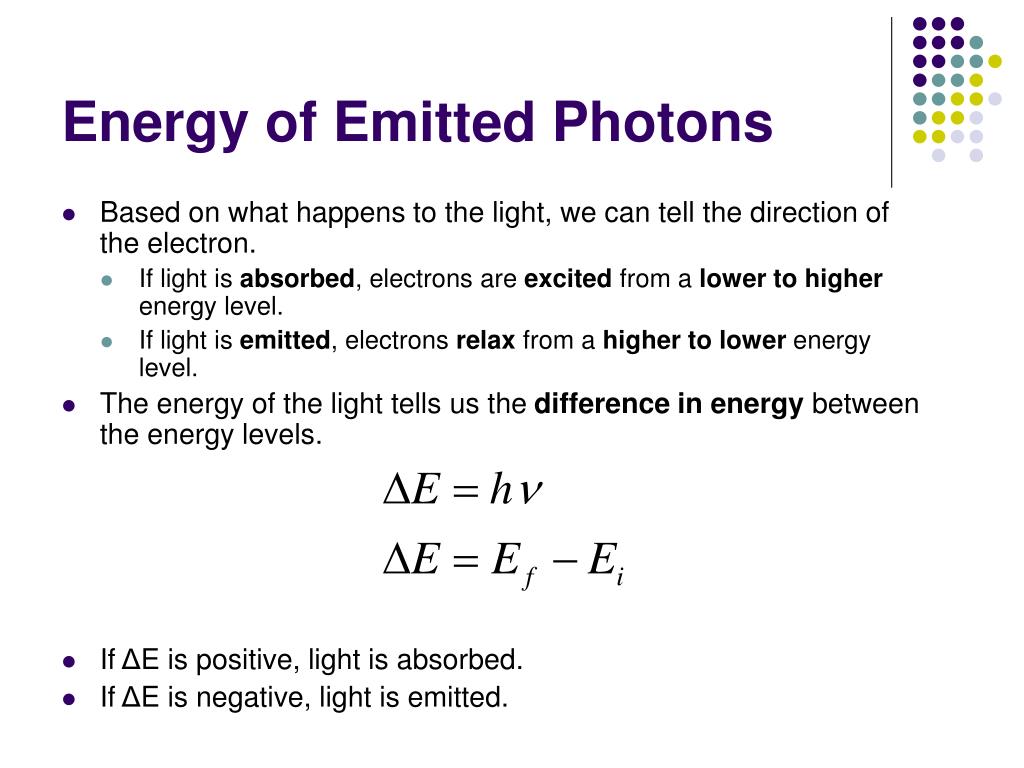
Photons Emitted From An Atom Appear As . If white light is passed through a sample of hydrogen, hydrogen. Atoms emit light when electrons in a higher energy orbital drop to a lower energy orbital. The energy of the emitted photon matches the energy lost by the electron. When the electrons of a certain atom return to lower orbitals from excited states, the photons they emit have energies that are characteristic of that kind of atom. When an atom in an excited state undergoes a transition to the ground state in a process called decay, it loses energy by. The photons emitted from an atom appear as blank #3. In my post about how we know photons exist, i make reference to the famous kimble, dagenais, and mandel experiment. Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic physicists to measure and. When an atom absorbs light, it is excited to a higher energy state. However, quantum optics tell us that. When an atom emits light, it decays to a lower energy state;
from www.slideserve.com
When an atom emits light, it decays to a lower energy state; Atoms emit light when electrons in a higher energy orbital drop to a lower energy orbital. In my post about how we know photons exist, i make reference to the famous kimble, dagenais, and mandel experiment. The energy of the emitted photon matches the energy lost by the electron. When an atom in an excited state undergoes a transition to the ground state in a process called decay, it loses energy by. If white light is passed through a sample of hydrogen, hydrogen. When an atom absorbs light, it is excited to a higher energy state. However, quantum optics tell us that. Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic physicists to measure and. The photons emitted from an atom appear as blank #3.
PPT Honors Chemistry Unit 3 Electrons PowerPoint Presentation, free download ID6524754
Photons Emitted From An Atom Appear As Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic physicists to measure and. In my post about how we know photons exist, i make reference to the famous kimble, dagenais, and mandel experiment. When an atom emits light, it decays to a lower energy state; The photons emitted from an atom appear as blank #3. When an atom absorbs light, it is excited to a higher energy state. However, quantum optics tell us that. The energy of the emitted photon matches the energy lost by the electron. When the electrons of a certain atom return to lower orbitals from excited states, the photons they emit have energies that are characteristic of that kind of atom. Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic physicists to measure and. When an atom in an excited state undergoes a transition to the ground state in a process called decay, it loses energy by. If white light is passed through a sample of hydrogen, hydrogen. Atoms emit light when electrons in a higher energy orbital drop to a lower energy orbital.
From www.numerade.com
SOLVED In a set of experiments on a hypothetical oneelectron atom, you measure the wavelengths Photons Emitted From An Atom Appear As The energy of the emitted photon matches the energy lost by the electron. Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic physicists to measure and. In my post about how we know photons exist, i make reference to the famous kimble,. Photons Emitted From An Atom Appear As.
From www.shanghai-optics.com
Lesson 14 How do Atoms Emit Light? Photons Emitted From An Atom Appear As Atoms emit light when electrons in a higher energy orbital drop to a lower energy orbital. When an atom absorbs light, it is excited to a higher energy state. However, quantum optics tell us that. Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way. Photons Emitted From An Atom Appear As.
From wizedu.com
An electron in the hydrogen atom falls from the 2p to 1s state and a photon is emitted. What is Photons Emitted From An Atom Appear As However, quantum optics tell us that. When an atom absorbs light, it is excited to a higher energy state. Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic physicists to measure and. The photons emitted from an atom appear as blank #3.. Photons Emitted From An Atom Appear As.
From www.shutterstock.com
Photon Energy Emitted Bohr Model Atomic Stock Vector (Royalty Free) 1967288638 Shutterstock Photons Emitted From An Atom Appear As The energy of the emitted photon matches the energy lost by the electron. In my post about how we know photons exist, i make reference to the famous kimble, dagenais, and mandel experiment. If white light is passed through a sample of hydrogen, hydrogen. However, quantum optics tell us that. When an atom in an excited state undergoes a transition. Photons Emitted From An Atom Appear As.
From wizedu.com
7. Suppose the number of photons emitted by an atom during a oneminute time window can... WizEdu Photons Emitted From An Atom Appear As When an atom absorbs light, it is excited to a higher energy state. In my post about how we know photons exist, i make reference to the famous kimble, dagenais, and mandel experiment. Atoms emit light when electrons in a higher energy orbital drop to a lower energy orbital. However, quantum optics tell us that. Absorbed and emitted photons can. Photons Emitted From An Atom Appear As.
From www.numerade.com
Calculate the energy of a photon emitted when an electron in a hydrogen atom undergoes a Photons Emitted From An Atom Appear As The photons emitted from an atom appear as blank #3. When an atom emits light, it decays to a lower energy state; When the electrons of a certain atom return to lower orbitals from excited states, the photons they emit have energies that are characteristic of that kind of atom. Atoms emit light when electrons in a higher energy orbital. Photons Emitted From An Atom Appear As.
From www.numerade.com
SOLVED 'A hydrogen atom in an excited state emits a photon of wavelength 95 nm. What are the Photons Emitted From An Atom Appear As The energy of the emitted photon matches the energy lost by the electron. However, quantum optics tell us that. The photons emitted from an atom appear as blank #3. When an atom emits light, it decays to a lower energy state; In my post about how we know photons exist, i make reference to the famous kimble, dagenais, and mandel. Photons Emitted From An Atom Appear As.
From www.youtube.com
Electron Energy Levels and Photons IB Physics YouTube Photons Emitted From An Atom Appear As The photons emitted from an atom appear as blank #3. If white light is passed through a sample of hydrogen, hydrogen. When an atom emits light, it decays to a lower energy state; The energy of the emitted photon matches the energy lost by the electron. Atoms emit light when electrons in a higher energy orbital drop to a lower. Photons Emitted From An Atom Appear As.
From www.slideserve.com
PPT Photon Tissue Interactions PowerPoint Presentation, free download ID628678 Photons Emitted From An Atom Appear As When the electrons of a certain atom return to lower orbitals from excited states, the photons they emit have energies that are characteristic of that kind of atom. Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic physicists to measure and. In. Photons Emitted From An Atom Appear As.
From www.toppr.com
A hydrogen atom emits a photon corresponding to an electron transition from n = 5 to n = 1 . The Photons Emitted From An Atom Appear As When an atom emits light, it decays to a lower energy state; Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic physicists to measure and. When the electrons of a certain atom return to lower orbitals from excited states, the photons they. Photons Emitted From An Atom Appear As.
From www.numerade.com
SOLVED A Photon is emitted when an electron from a hydrogen atom undergoes a transition from a Photons Emitted From An Atom Appear As When an atom emits light, it decays to a lower energy state; The photons emitted from an atom appear as blank #3. When an atom in an excited state undergoes a transition to the ground state in a process called decay, it loses energy by. The energy of the emitted photon matches the energy lost by the electron. When an. Photons Emitted From An Atom Appear As.
From courses.lumenlearning.com
Photon Momentum Physics Photons Emitted From An Atom Appear As Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic physicists to measure and. When the electrons of a certain atom return to lower orbitals from excited states, the photons they emit have energies that are characteristic of that kind of atom. When. Photons Emitted From An Atom Appear As.
From www.slideserve.com
PPT Electrons in Atoms PowerPoint Presentation, free download ID2061071 Photons Emitted From An Atom Appear As When an atom absorbs light, it is excited to a higher energy state. When an atom emits light, it decays to a lower energy state; The photons emitted from an atom appear as blank #3. If white light is passed through a sample of hydrogen, hydrogen. The energy of the emitted photon matches the energy lost by the electron. Atoms. Photons Emitted From An Atom Appear As.
From saylordotorg.github.io
Atomic Spectra and Models of the Atom Photons Emitted From An Atom Appear As When an atom absorbs light, it is excited to a higher energy state. In my post about how we know photons exist, i make reference to the famous kimble, dagenais, and mandel experiment. Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic. Photons Emitted From An Atom Appear As.
From www.scienceabc.com
Where Do The Photons Produced By A Source Of Light Come From? » ScienceABC Photons Emitted From An Atom Appear As When an atom in an excited state undergoes a transition to the ground state in a process called decay, it loses energy by. When the electrons of a certain atom return to lower orbitals from excited states, the photons they emit have energies that are characteristic of that kind of atom. However, quantum optics tell us that. Atoms emit light. Photons Emitted From An Atom Appear As.
From www.numerade.com
SOLVED Part A For hydrogen, what is the wavelength of the photon emitted when an electron drops Photons Emitted From An Atom Appear As When an atom absorbs light, it is excited to a higher energy state. In my post about how we know photons exist, i make reference to the famous kimble, dagenais, and mandel experiment. The energy of the emitted photon matches the energy lost by the electron. The photons emitted from an atom appear as blank #3. If white light is. Photons Emitted From An Atom Appear As.
From byjus.com
a hydrogen atom at rest emits a photon in going from n=5 to n=1 state, then recoil speed of atom Photons Emitted From An Atom Appear As The photons emitted from an atom appear as blank #3. Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic physicists to measure and. When an atom emits light, it decays to a lower energy state; The energy of the emitted photon matches. Photons Emitted From An Atom Appear As.
From physics.aps.org
Physics Viewpoint Single Dot Meets Single Ion Photons Emitted From An Atom Appear As However, quantum optics tell us that. When an atom emits light, it decays to a lower energy state; If white light is passed through a sample of hydrogen, hydrogen. When an atom absorbs light, it is excited to a higher energy state. The energy of the emitted photon matches the energy lost by the electron. Atoms emit light when electrons. Photons Emitted From An Atom Appear As.
From www.doubtnut.com
The wavelength of the photon emitted by a hydrogen atom when an electron makes a transition from Photons Emitted From An Atom Appear As When the electrons of a certain atom return to lower orbitals from excited states, the photons they emit have energies that are characteristic of that kind of atom. Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic physicists to measure and. Atoms. Photons Emitted From An Atom Appear As.
From education-portal.com
Electron Transitions & Spectral Lines Video & Lesson Transcript Photons Emitted From An Atom Appear As The photons emitted from an atom appear as blank #3. When an atom absorbs light, it is excited to a higher energy state. When the electrons of a certain atom return to lower orbitals from excited states, the photons they emit have energies that are characteristic of that kind of atom. Absorbed and emitted photons can reveal key information about. Photons Emitted From An Atom Appear As.
From www.youtube.com
Calculate the wavelength of the photon emitted when the hydrogen atom transition from n=5 to n=3 Photons Emitted From An Atom Appear As When the electrons of a certain atom return to lower orbitals from excited states, the photons they emit have energies that are characteristic of that kind of atom. When an atom in an excited state undergoes a transition to the ground state in a process called decay, it loses energy by. However, quantum optics tell us that. If white light. Photons Emitted From An Atom Appear As.
From www.researchgate.net
(PDF) Nonlocal property of singlephoton states Illustration with spontaneously emitted photon Photons Emitted From An Atom Appear As Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic physicists to measure and. The energy of the emitted photon matches the energy lost by the electron. When an atom in an excited state undergoes a transition to the ground state in a. Photons Emitted From An Atom Appear As.
From www.slideserve.com
PPT Honors Chemistry Unit 3 Electrons PowerPoint Presentation, free download ID6524754 Photons Emitted From An Atom Appear As When an atom in an excited state undergoes a transition to the ground state in a process called decay, it loses energy by. When an atom emits light, it decays to a lower energy state; When an atom absorbs light, it is excited to a higher energy state. In my post about how we know photons exist, i make reference. Photons Emitted From An Atom Appear As.
From www.researchgate.net
Electron energy levels K, L, M, N in hydrogen atom. Emitted photons are... Download Scientific Photons Emitted From An Atom Appear As Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic physicists to measure and. The photons emitted from an atom appear as blank #3. When an atom in an excited state undergoes a transition to the ground state in a process called decay,. Photons Emitted From An Atom Appear As.
From schoolbag.info
Rutherford, Planck, and Bohr Atomic Structure Review Training MCAT General Chemistry Review Photons Emitted From An Atom Appear As If white light is passed through a sample of hydrogen, hydrogen. Atoms emit light when electrons in a higher energy orbital drop to a lower energy orbital. When an atom in an excited state undergoes a transition to the ground state in a process called decay, it loses energy by. When the electrons of a certain atom return to lower. Photons Emitted From An Atom Appear As.
From slideplayer.com
The Spectrum and Blackbody Radiation ppt download Photons Emitted From An Atom Appear As When an atom absorbs light, it is excited to a higher energy state. Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic physicists to measure and. When the electrons of a certain atom return to lower orbitals from excited states, the photons. Photons Emitted From An Atom Appear As.
From www.toppr.com
What is the wavelength of a photon emitted during a transition from n = 5 state to n = 2 state Photons Emitted From An Atom Appear As When an atom emits light, it decays to a lower energy state; When an atom in an excited state undergoes a transition to the ground state in a process called decay, it loses energy by. If white light is passed through a sample of hydrogen, hydrogen. In my post about how we know photons exist, i make reference to the. Photons Emitted From An Atom Appear As.
From www.slideserve.com
PPT Chapter 42 PowerPoint Presentation, free download ID5580472 Photons Emitted From An Atom Appear As When the electrons of a certain atom return to lower orbitals from excited states, the photons they emit have energies that are characteristic of that kind of atom. The photons emitted from an atom appear as blank #3. Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is. Photons Emitted From An Atom Appear As.
From www.slideshare.net
The Atom & Spectra Photons Emitted From An Atom Appear As However, quantum optics tell us that. In my post about how we know photons exist, i make reference to the famous kimble, dagenais, and mandel experiment. When an atom emits light, it decays to a lower energy state; When an atom absorbs light, it is excited to a higher energy state. The energy of the emitted photon matches the energy. Photons Emitted From An Atom Appear As.
From www.toppr.com
The wavelength of photon emitted when an electron jumps from a 4d orbital to a 2p orbital in Photons Emitted From An Atom Appear As Atoms emit light when electrons in a higher energy orbital drop to a lower energy orbital. When an atom absorbs light, it is excited to a higher energy state. In my post about how we know photons exist, i make reference to the famous kimble, dagenais, and mandel experiment. Absorbed and emitted photons can reveal key information about the state. Photons Emitted From An Atom Appear As.
From www.researchgate.net
Stimulated emission. When a photon hits an excited atom, two photons... Download Scientific Photons Emitted From An Atom Appear As Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic physicists to measure and. When the electrons of a certain atom return to lower orbitals from excited states, the photons they emit have energies that are characteristic of that kind of atom. If. Photons Emitted From An Atom Appear As.
From pages.uoregon.edu
Astronomy 122 Spectroscopy Photons Emitted From An Atom Appear As The energy of the emitted photon matches the energy lost by the electron. When an atom emits light, it decays to a lower energy state; Absorbed and emitted photons can reveal key information about the state of an atom and its electrons, and examining this light is a common way for atomic physicists to measure and. However, quantum optics tell. Photons Emitted From An Atom Appear As.
From www.youtube.com
ABC Zoom Electrons and photons absorption and transmission of light YouTube Photons Emitted From An Atom Appear As When an atom emits light, it decays to a lower energy state; When the electrons of a certain atom return to lower orbitals from excited states, the photons they emit have energies that are characteristic of that kind of atom. When an atom absorbs light, it is excited to a higher energy state. When an atom in an excited state. Photons Emitted From An Atom Appear As.
From www.slideserve.com
PPT Matter and Particles of Light Quantum Theory PowerPoint Presentation ID672824 Photons Emitted From An Atom Appear As The photons emitted from an atom appear as blank #3. In my post about how we know photons exist, i make reference to the famous kimble, dagenais, and mandel experiment. If white light is passed through a sample of hydrogen, hydrogen. When an atom absorbs light, it is excited to a higher energy state. However, quantum optics tell us that.. Photons Emitted From An Atom Appear As.
From www.eurekalert.org
A Photon Is Emitted and Reabso [IMAGE] EurekAlert! Science News Releases Photons Emitted From An Atom Appear As If white light is passed through a sample of hydrogen, hydrogen. When an atom emits light, it decays to a lower energy state; Atoms emit light when electrons in a higher energy orbital drop to a lower energy orbital. The photons emitted from an atom appear as blank #3. Absorbed and emitted photons can reveal key information about the state. Photons Emitted From An Atom Appear As.