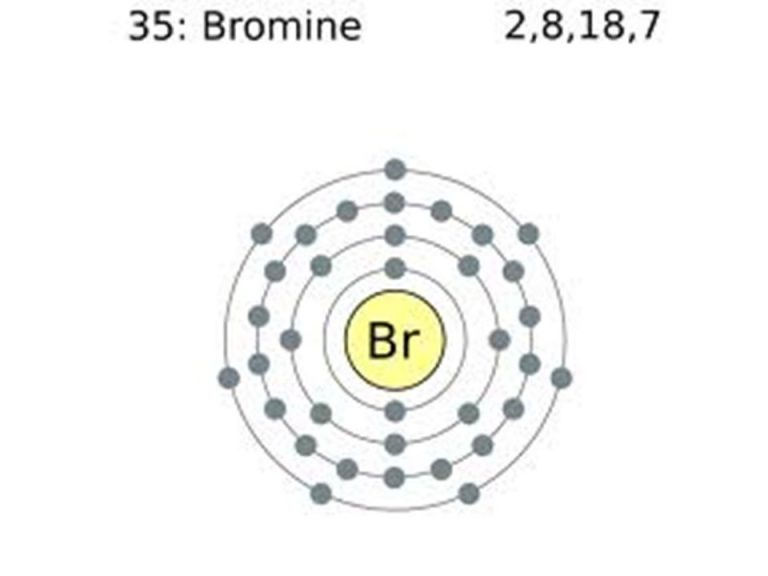
Bromine Electron Configuration Explanation . Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. Now we discuss what is the electron configuration of bromine.the electron configuration of bromine is [ar] 4s² 3d¹⁰ 4p⁵. Bromine (br) is a chemical element. Use a chart such as the one. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. This can be shortened to [ar]4s23d104p5. The atomic number of bromine is 35. Since bromine has 7 valence. Melting point the temperature at which the. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. To understand this principle, let's consider the bromine atom.
from periodictable.me
Now we discuss what is the electron configuration of bromine.the electron configuration of bromine is [ar] 4s² 3d¹⁰ 4p⁵. Bromine (br) is a chemical element. Since bromine has 7 valence. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. To understand this principle, let's consider the bromine atom. Melting point the temperature at which the. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. This can be shortened to [ar]4s23d104p5. The atomic number of bromine is 35. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5.
Bromine Electron Configuration (Br) with Orbital Diagram
Bromine Electron Configuration Explanation To understand this principle, let's consider the bromine atom. Use a chart such as the one. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Bromine (br) is a chemical element. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Melting point the temperature at which the. Since bromine has 7 valence. Now we discuss what is the electron configuration of bromine.the electron configuration of bromine is [ar] 4s² 3d¹⁰ 4p⁵. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. To understand this principle, let's consider the bromine atom. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The atomic number of bromine is 35. This can be shortened to [ar]4s23d104p5.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Configuration Explanation Now we discuss what is the electron configuration of bromine.the electron configuration of bromine is [ar] 4s² 3d¹⁰ 4p⁵. Bromine (br) is a chemical element. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. This can be shortened to [ar]4s23d104p5. Since bromine has 7 valence. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s. Bromine Electron Configuration Explanation.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Configuration Explanation The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Use a chart such as the one. Bromine (z=35), which has 35 electrons, can be found. Bromine Electron Configuration Explanation.
From www.numerade.com
SOLVEDWhat is the electron configuration of a bromine atom (You CAN Bromine Electron Configuration Explanation The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Bromine (br) is a chemical element. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p. Bromine Electron Configuration Explanation.
From www.aiohotzgirl.com
Electron Configuration Bromine Chemical Element Electron Shell Bohr Bromine Electron Configuration Explanation Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. To understand this principle, let's consider the bromine atom. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases. Bromine Electron Configuration Explanation.
From www.slideserve.com
PPT Draw Bromine Electron Shell Configuration 1s 2 2s 2 2p 6 3s 2 3p Bromine Electron Configuration Explanation Use a chart such as the one. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. Bromine (br) is a chemical element. Melting point the temperature at which the. Since bromine has 7 valence. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of. Bromine Electron Configuration Explanation.
From ar.inspiredpencil.com
Bromine Electron Configuration Bromine Electron Configuration Explanation The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. This can be shortened to [ar]4s23d104p5. To understand this principle, let's consider the bromine atom. Now we discuss what is the electron configuration. Bromine Electron Configuration Explanation.
From ar.inspiredpencil.com
Electron Configuration For Bromine Bromine Electron Configuration Explanation The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. This can be shortened to [ar]4s23d104p5. The atomic number of bromine is 35. To understand this principle, let's consider the bromine atom. Since bromine has 7 valence. Now we discuss what is the electron configuration of bromine.the electron configuration of bromine is [ar] 4s² 3d¹⁰ 4p⁵. Bromine (z=35), which has 35 electrons, can. Bromine Electron Configuration Explanation.
From www.alamy.com
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy Bromine Electron Configuration Explanation Now we discuss what is the electron configuration of bromine.the electron configuration of bromine is [ar] 4s² 3d¹⁰ 4p⁵. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. To understand this principle, let's consider the bromine atom. The atomic number of bromine is 35. Melting point the temperature at. Bromine Electron Configuration Explanation.
From www.numerade.com
SOLVEDUse electron configurations to explain why (a) sulfur has a Bromine Electron Configuration Explanation To understand this principle, let's consider the bromine atom. Use a chart such as the one. The atomic number of bromine is 35. Since bromine has 7 valence. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the. Bromine Electron Configuration Explanation.
From socratic.org
Electronic configuration of bromine ? Socratic Bromine Electron Configuration Explanation Now we discuss what is the electron configuration of bromine.the electron configuration of bromine is [ar] 4s² 3d¹⁰ 4p⁵. Since bromine has 7 valence. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The bromine electron configuration, denoted as [ar] 4s 2 3d 10. Bromine Electron Configuration Explanation.
From ar.inspiredpencil.com
Bromine Model Bromine Electron Configuration Explanation Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Use a chart. Bromine Electron Configuration Explanation.
From ar.inspiredpencil.com
Electron Configuration For Bromine Bromine Electron Configuration Explanation Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The atomic number of bromine is 35. Since bromine has 7 valence. This can be shortened to [ar]4s23d104p5. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. The bromine electron configuration, denoted as [ar] 4s 2. Bromine Electron Configuration Explanation.
From resource.studiaacademy.com
2.2 Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Bromine Electron Configuration Explanation Since bromine has 7 valence. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Bromine (br) is a chemical element. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Now we discuss what is. Bromine Electron Configuration Explanation.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Electron Configuration Explanation Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. This can be shortened to [ar]4s23d104p5. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement. Bromine Electron Configuration Explanation.
From www.numerade.com
SOLVED While the electron affinity of bromine is a negative quantity Bromine Electron Configuration Explanation Bromine (br) is a chemical element. To understand this principle, let's consider the bromine atom. Now we discuss what is the electron configuration of bromine.the electron configuration of bromine is [ar] 4s² 3d¹⁰ 4p⁵. The atomic number of bromine is 35. This can be shortened to [ar]4s23d104p5. Bromine is in the 4th energy level, p block, 5th column, this means. Bromine Electron Configuration Explanation.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 2.2.2 Group 7 Reactivity & Electronic Bromine Electron Configuration Explanation This can be shortened to [ar]4s23d104p5. Melting point the temperature at which the. Now we discuss what is the electron configuration of bromine.the electron configuration of bromine is [ar] 4s² 3d¹⁰ 4p⁵. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. Bromine (br) is a chemical element. The electron. Bromine Electron Configuration Explanation.
From socratic.org
Why does fluorine have a higher ionization energy than bromine? Socratic Bromine Electron Configuration Explanation Bromine (br) is a chemical element. Melting point the temperature at which the. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p. Bromine Electron Configuration Explanation.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Bromine Electron Configuration Explanation The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Bromine (br) is. Bromine Electron Configuration Explanation.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Configuration Explanation Bromine (br) is a chemical element. Now we discuss what is the electron configuration of bromine.the electron configuration of bromine is [ar] 4s² 3d¹⁰ 4p⁵. This can be shortened to [ar]4s23d104p5. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. Melting point the temperature. Bromine Electron Configuration Explanation.
From brainly.com
Which is the electron configuration for bromine? Bromine Electron Configuration Explanation The atomic number of bromine is 35. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Melting point the temperature at which the. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p. Bromine Electron Configuration Explanation.
From www.numerade.com
SOLVEDAlthough the electron affinity of bromine is a negative quantity Bromine Electron Configuration Explanation The atomic number of bromine is 35. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. Since bromine has 7 valence. Use a chart such as the one. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p. Bromine Electron Configuration Explanation.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Configuration Explanation Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. Now we discuss what is the electron configuration of bromine.the electron configuration of bromine is [ar] 4s² 3d¹⁰ 4p⁵. Since bromine has 7 valence. This can be shortened to [ar]4s23d104p5. To understand this principle, let's consider the bromine atom. Melting. Bromine Electron Configuration Explanation.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromine Electron Configuration Explanation Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Since bromine has. Bromine Electron Configuration Explanation.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Electron Configuration Explanation Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. Since bromine has 7 valence. Melting point the temperature at which the. Use a chart such as the one. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Bromine. Bromine Electron Configuration Explanation.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine Electron Configuration Explanation Electron configuration the arrangements of electrons above the last (closed shell) noble gas. This can be shortened to [ar]4s23d104p5. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. Now we discuss what is the electron configuration of bromine.the electron configuration of bromine is [ar] 4s² 3d¹⁰ 4p⁵. The bromine. Bromine Electron Configuration Explanation.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Bromine Electron Configuration Explanation Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Melting point the temperature at which the. This can be shortened to [ar]4s23d104p5. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or. Bromine Electron Configuration Explanation.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Configuration Explanation Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. The electron configuration. Bromine Electron Configuration Explanation.
From keplarllp.com
😍 Electron configuration examples. Abbreviated Electron configurations Bromine Electron Configuration Explanation Bromine (br) is a chemical element. Now we discuss what is the electron configuration of bromine.the electron configuration of bromine is [ar] 4s² 3d¹⁰ 4p⁵. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Melting point the temperature at which the. Since bromine has 7 valence. Bromine (z=35), which has 35 electrons, can be found in. Bromine Electron Configuration Explanation.
From ar.inspiredpencil.com
Electron Configuration For Bromine Bromine Electron Configuration Explanation Bromine (br) is a chemical element. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. To understand this principle, let's consider the bromine atom. This can be shortened to [ar]4s23d104p5. Use a chart such as the one. Since bromine has 7 valence. The atomic number of bromine is 35.. Bromine Electron Configuration Explanation.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Configuration Explanation Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The atomic number of bromine is 35. Melting point the temperature at which the. Now we discuss what is the electron configuration of bromine.the electron configuration of bromine is [ar] 4s² 3d¹⁰ 4p⁵. Bromine (z=35), which has 35 electrons, can. Bromine Electron Configuration Explanation.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Configuration Explanation The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Since bromine has 7 valence. The atomic number of bromine is 35. Melting point the temperature at which the. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. Bromine. Bromine Electron Configuration Explanation.
From ar.inspiredpencil.com
Electron Configuration Of Bromine Bromine Electron Configuration Explanation To understand this principle, let's consider the bromine atom. Bromine (br) is a chemical element. Now we discuss what is the electron configuration of bromine.the electron configuration of bromine is [ar] 4s² 3d¹⁰ 4p⁵. Use a chart such as the one. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. The. Bromine Electron Configuration Explanation.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Electron Configuration Explanation The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. Use a chart such as the one. Bromine is in the 4th energy level, p block, 5th column, this means that the electron configuration will end 4p5. Now we discuss what is the electron configuration. Bromine Electron Configuration Explanation.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Configuration Explanation Melting point the temperature at which the. Now we discuss what is the electron configuration of bromine.the electron configuration of bromine is [ar] 4s² 3d¹⁰ 4p⁵. To understand this principle, let's consider the bromine atom. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. The atomic number of bromine is 35. The electron configuration of bromine. Bromine Electron Configuration Explanation.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Electron Configuration Explanation Bromine (z=35), which has 35 electrons, can be found in period 4, group vii of the periodic table. The atomic number of bromine is 35. Melting point the temperature at which the. Bromine (br) is a chemical element. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5 or 1s. Bromine Electron Configuration Explanation.