Acid Base Titration Lab Answers Quizlet . To standardize a solution of base such as naoh, an acid whose amount can be determined to a high degree of accuracy as a primary standard is needed. A technique for determining the amount of a certain substance by doing a titration. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Find out how to choose the right indicator,. Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar reactions in. Follow the procedure, calculate the concentration of naoh, and compare fast and slow titrations. Terms in this set (14) volumetric analysis. Study with quizlet and memorize flashcards containing terms like what is the objective?, formula of acetic acid, formula of. Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more.
from www.chegg.com
Find out how to choose the right indicator,. Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar reactions in. Terms in this set (14) volumetric analysis. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. A technique for determining the amount of a certain substance by doing a titration. To standardize a solution of base such as naoh, an acid whose amount can be determined to a high degree of accuracy as a primary standard is needed. Follow the procedure, calculate the concentration of naoh, and compare fast and slow titrations. Study with quizlet and memorize flashcards containing terms like what is the objective?, formula of acetic acid, formula of. Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more.
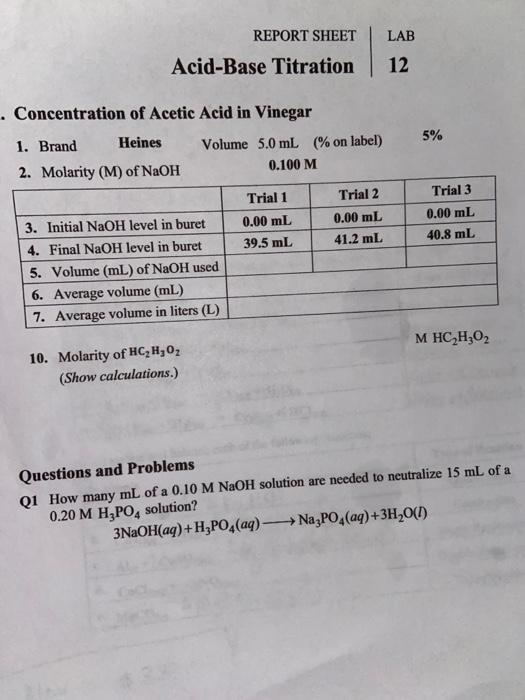
Solved LAB REPORT SHEET AcidBase Titration 12 .
Acid Base Titration Lab Answers Quizlet Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar reactions in. To standardize a solution of base such as naoh, an acid whose amount can be determined to a high degree of accuracy as a primary standard is needed. Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Follow the procedure, calculate the concentration of naoh, and compare fast and slow titrations. Study with quizlet and memorize flashcards containing terms like what is the objective?, formula of acetic acid, formula of. Find out how to choose the right indicator,. Terms in this set (14) volumetric analysis. A technique for determining the amount of a certain substance by doing a titration. Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar reactions in.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration Acid Base Titration Lab Answers Quizlet Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar reactions in. Terms in this set (14) volumetric analysis. A technique for determining the amount of a certain substance by doing a titration. Follow the procedure, calculate the concentration of naoh, and compare fast and slow titrations. Study with quizlet and memorize. Acid Base Titration Lab Answers Quizlet.
From www.docsity.com
Titration Practice Acid Base Reaction Worksheet with Answer Key Docsity Acid Base Titration Lab Answers Quizlet Study with quizlet and memorize flashcards containing terms like what is the objective?, formula of acetic acid, formula of. Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. To standardize a solution of base such as naoh, an acid whose amount can be determined to a high degree of accuracy as. Acid Base Titration Lab Answers Quizlet.
From eduvibecentral.com
Decoding the Complexities of AcidBase Titration Lab Answers in PDF Format Acid Base Titration Lab Answers Quizlet A technique for determining the amount of a certain substance by doing a titration. To standardize a solution of base such as naoh, an acid whose amount can be determined to a high degree of accuracy as a primary standard is needed. Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar. Acid Base Titration Lab Answers Quizlet.
From www.docsity.com
Acid base titration lab answers Docsity Acid Base Titration Lab Answers Quizlet To standardize a solution of base such as naoh, an acid whose amount can be determined to a high degree of accuracy as a primary standard is needed. Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar reactions in. A technique for determining the amount of a certain substance by doing. Acid Base Titration Lab Answers Quizlet.
From melanie-chapter.blogspot.com
Titration Of Acids And Bases Lab Report Experiment 20 Answers 45+ Pages Explanation Doc [1.6mb Acid Base Titration Lab Answers Quizlet A technique for determining the amount of a certain substance by doing a titration. Terms in this set (14) volumetric analysis. Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. Find out how to choose the right indicator,. Study with quizlet and memorize flashcards containing terms like what is the objective?,. Acid Base Titration Lab Answers Quizlet.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Acid Base Titration Lab Answers Quizlet Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. Terms in this set (14) volumetric analysis. Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar reactions in. Follow the procedure, calculate the concentration of naoh, and compare fast and slow titrations. Study. Acid Base Titration Lab Answers Quizlet.
From www.studypool.com
SOLUTION CHM 113 Experiment 8 Acid Base Titration Lab Report Studypool Acid Base Titration Lab Answers Quizlet Follow the procedure, calculate the concentration of naoh, and compare fast and slow titrations. Terms in this set (14) volumetric analysis. A technique for determining the amount of a certain substance by doing a titration. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Study with quizlet and. Acid Base Titration Lab Answers Quizlet.
From www.studypool.com
SOLUTION CHM 113 Experiment 8 Acid Base Titration Lab Report Studypool Acid Base Titration Lab Answers Quizlet To standardize a solution of base such as naoh, an acid whose amount can be determined to a high degree of accuracy as a primary standard is needed. Terms in this set (14) volumetric analysis. A technique for determining the amount of a certain substance by doing a titration. Learn how to perform a titration to determine the concentration of. Acid Base Titration Lab Answers Quizlet.
From www.chegg.com
Solved REPORT SHEET AcidBase Titration LAB 20 1. Brand Acid Base Titration Lab Answers Quizlet Terms in this set (14) volumetric analysis. Follow the procedure, calculate the concentration of naoh, and compare fast and slow titrations. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Find out how to choose the right indicator,. Study with quizlet and memorize flashcards containing terms like what. Acid Base Titration Lab Answers Quizlet.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Acid Base Titration Lab Answers Quizlet A technique for determining the amount of a certain substance by doing a titration. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Study with quizlet and memorize flashcards containing terms like what is the objective?, formula of acetic acid, formula of. Terms in this set (14) volumetric. Acid Base Titration Lab Answers Quizlet.
From mungfali.com
Acid Base Titration Lab Acid Base Titration Lab Answers Quizlet Follow the procedure, calculate the concentration of naoh, and compare fast and slow titrations. Terms in this set (14) volumetric analysis. Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar reactions in. Study with quizlet and memorize flashcards containing terms like what is the objective?, formula of acetic acid, formula of.. Acid Base Titration Lab Answers Quizlet.
From soetrust.org
2022 UPDATED!!! Acid base titration lab answers Soetrust Acid Base Titration Lab Answers Quizlet Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. A technique for determining the amount of a certain substance by doing a titration. To standardize a solution of base such as naoh, an acid whose amount can be determined to a high degree of accuracy as a primary standard is needed.. Acid Base Titration Lab Answers Quizlet.
From www.vrogue.co
Acid Base Titration Lab Questions Answers vrogue.co Acid Base Titration Lab Answers Quizlet Follow the procedure, calculate the concentration of naoh, and compare fast and slow titrations. Find out how to choose the right indicator,. A technique for determining the amount of a certain substance by doing a titration. Study with quizlet and memorize flashcards containing terms like what is the objective?, formula of acetic acid, formula of. Terms in this set (14). Acid Base Titration Lab Answers Quizlet.
From www.chegg.com
Solved AcidBase Titration Virtual Lab Introduction In Acid Base Titration Lab Answers Quizlet Find out how to choose the right indicator,. Follow the procedure, calculate the concentration of naoh, and compare fast and slow titrations. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Terms in this set (14) volumetric analysis. Study with quizlet and memorize flashcards containing terms like objective,. Acid Base Titration Lab Answers Quizlet.
From mungfali.com
Acid Base Titration Experiment Acid Base Titration Lab Answers Quizlet Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar reactions in. Study with quizlet and memorize flashcards containing terms like what is the objective?, formula of acetic acid, formula of. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator.. Acid Base Titration Lab Answers Quizlet.
From www.studypool.com
SOLUTION Lab 6 acid base titrations Studypool Acid Base Titration Lab Answers Quizlet A technique for determining the amount of a certain substance by doing a titration. To standardize a solution of base such as naoh, an acid whose amount can be determined to a high degree of accuracy as a primary standard is needed. Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar. Acid Base Titration Lab Answers Quizlet.
From ebinu.blog
AcidBase Titration Lab — DataClassroom / Acid Base Titration Chemistry 1210 Lab report Acid Base Titration Lab Answers Quizlet Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. To standardize a solution of base such as naoh, an acid whose amount can be determined to a high degree of accuracy as a primary standard is needed. Study with quizlet and memorize flashcards containing terms like what is. Acid Base Titration Lab Answers Quizlet.
From www.coursehero.com
[Solved] Lab report on Acid base titration Course Hero Acid Base Titration Lab Answers Quizlet Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar reactions in. Terms in this set (14) volumetric analysis. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Find out how to choose the right indicator,. Study with quizlet and. Acid Base Titration Lab Answers Quizlet.
From www.chegg.com
Solved REPORT SHEET LAB AcidBase Titration 20 A. Acid Base Titration Lab Answers Quizlet A technique for determining the amount of a certain substance by doing a titration. Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar reactions in. Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. Learn how to perform a titration to determine. Acid Base Titration Lab Answers Quizlet.
From www.vrogue.co
Acid Base Titration Worksheet Answers Acid Base Titra vrogue.co Acid Base Titration Lab Answers Quizlet A technique for determining the amount of a certain substance by doing a titration. Follow the procedure, calculate the concentration of naoh, and compare fast and slow titrations. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Study with quizlet and memorize flashcards containing terms like objective, purpose,. Acid Base Titration Lab Answers Quizlet.
From studyfinder.org
Unveiling Acid Base Titration Pre Lab Answers A Comprehensive Guide Acid Base Titration Lab Answers Quizlet To standardize a solution of base such as naoh, an acid whose amount can be determined to a high degree of accuracy as a primary standard is needed. Terms in this set (14) volumetric analysis. Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar reactions in. Study with quizlet and memorize. Acid Base Titration Lab Answers Quizlet.
From eduvibecentral.com
Decoding the Complexities of AcidBase Titration Lab Answers in PDF Format Acid Base Titration Lab Answers Quizlet Follow the procedure, calculate the concentration of naoh, and compare fast and slow titrations. Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. Terms in this set (14) volumetric analysis. A technique for determining the amount of a certain substance by doing a titration. Study with quizlet and memorize flashcards containing. Acid Base Titration Lab Answers Quizlet.
From eduvibecentral.com
Decoding the Complexities of AcidBase Titration Lab Answers in PDF Format Acid Base Titration Lab Answers Quizlet Follow the procedure, calculate the concentration of naoh, and compare fast and slow titrations. Find out how to choose the right indicator,. Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar reactions in.. Acid Base Titration Lab Answers Quizlet.
From www.vrogue.co
Acid Base Titration Experiment And Phases Of Color Ch vrogue.co Acid Base Titration Lab Answers Quizlet Study with quizlet and memorize flashcards containing terms like what is the objective?, formula of acetic acid, formula of. To standardize a solution of base such as naoh, an acid whose amount can be determined to a high degree of accuracy as a primary standard is needed. Learn how to perform a titration to determine the concentration of an acid. Acid Base Titration Lab Answers Quizlet.
From www.vrogue.co
Acid Base Titration Lab Answers Answerdata vrogue.co Acid Base Titration Lab Answers Quizlet Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Follow the procedure, calculate the concentration of naoh, and compare fast and slow titrations. Find out how to choose the right. Acid Base Titration Lab Answers Quizlet.
From studylib.net
Acid/Base Titration Lab Acid Base Titration Lab Answers Quizlet A technique for determining the amount of a certain substance by doing a titration. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Follow the procedure, calculate the concentration of naoh, and compare fast and slow titrations. Study with quizlet and memorize flashcards containing terms like purpose of. Acid Base Titration Lab Answers Quizlet.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations, Examples, Solution Stoichiometry Acid Base Titration Lab Answers Quizlet Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar reactions in. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. A technique for determining the amount of a certain substance by doing a titration. Follow the procedure, calculate the. Acid Base Titration Lab Answers Quizlet.
From www.nagwa.com
Question Video Explaining Why the Conical Flask Is Placed on a White Tile in an AcidBase Acid Base Titration Lab Answers Quizlet A technique for determining the amount of a certain substance by doing a titration. Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar reactions in. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Study with quizlet and memorize. Acid Base Titration Lab Answers Quizlet.
From quizlet.com
AcidBase Titration Lab Quiz Flashcards Quizlet Acid Base Titration Lab Answers Quizlet A technique for determining the amount of a certain substance by doing a titration. To standardize a solution of base such as naoh, an acid whose amount can be determined to a high degree of accuracy as a primary standard is needed. Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar. Acid Base Titration Lab Answers Quizlet.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 12 . Acid Base Titration Lab Answers Quizlet Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Study with quizlet and memorize flashcards containing terms like what is the objective?, formula of acetic acid, formula of. Study with. Acid Base Titration Lab Answers Quizlet.
From avery-yersbloglester.blogspot.com
Acid Base Titration Lab Report Answers Acid Base Titration Lab Answers Quizlet Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. To standardize a solution of base such as naoh, an acid whose amount can be determined to a high degree of. Acid Base Titration Lab Answers Quizlet.
From moniquearesmeza.blogspot.com
Acid Base Titration Lab Report Answers MoniquearesMeza Acid Base Titration Lab Answers Quizlet A technique for determining the amount of a certain substance by doing a titration. To standardize a solution of base such as naoh, an acid whose amount can be determined to a high degree of accuracy as a primary standard is needed. Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more.. Acid Base Titration Lab Answers Quizlet.
From www.studocu.com
Lecture Notes 9 + Experiment 9 ACIDBASE TITRATION Experiment 9 AcidBase Titration Acid Base Titration Lab Answers Quizlet A technique for determining the amount of a certain substance by doing a titration. Study with quizlet and memorize flashcards containing terms like objective, purpose, one of the most common and familiar reactions in. Terms in this set (14) volumetric analysis. To standardize a solution of base such as naoh, an acid whose amount can be determined to a high. Acid Base Titration Lab Answers Quizlet.
From mateo.perka.org
Acid Base Titration Lab Answers mateo Acid Base Titration Lab Answers Quizlet A technique for determining the amount of a certain substance by doing a titration. Follow the procedure, calculate the concentration of naoh, and compare fast and slow titrations. To standardize a solution of base such as naoh, an acid whose amount can be determined to a high degree of accuracy as a primary standard is needed. Learn how to perform. Acid Base Titration Lab Answers Quizlet.
From yazminewadiaz.blogspot.com
Acid Base Titration Lab Report Answers YazminewaDiaz Acid Base Titration Lab Answers Quizlet Study with quizlet and memorize flashcards containing terms like purpose of this lab (6), monoprotic, diprotic and more. Find out how to choose the right indicator,. Learn how to perform a titration to determine the concentration of an acid or a base using phenolphthalein as an indicator. Study with quizlet and memorize flashcards containing terms like objective, purpose, one of. Acid Base Titration Lab Answers Quizlet.