Dilution Technical Definition . Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Serial dilutions are used in microbiology to reduce bacterial. Dilution is the process of reducing the concentration of a solute in a solution, usually by adding more solvent. It provides information that is of more practical benefit than recovery as it. Dilution is the process of weakening or deconcentrating a solution. For example, we might say that a glass. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Explanations and examples of common methods. This concept is crucial in. Dilution is a useful additional check on the accuracy of an assay. There are many ways of expressing concentration and dilution. Concentration is the removal of. You can add water to concentrated orange juice to.
from labpedia.net
It provides information that is of more practical benefit than recovery as it. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Dilution is the process of reducing the concentration of a solute in a solution, usually by adding more solvent. Serial dilutions are used in microbiology to reduce bacterial. Explanations and examples of common methods. Dilution is a useful additional check on the accuracy of an assay. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. This concept is crucial in. You can add water to concentrated orange juice to. There are many ways of expressing concentration and dilution.
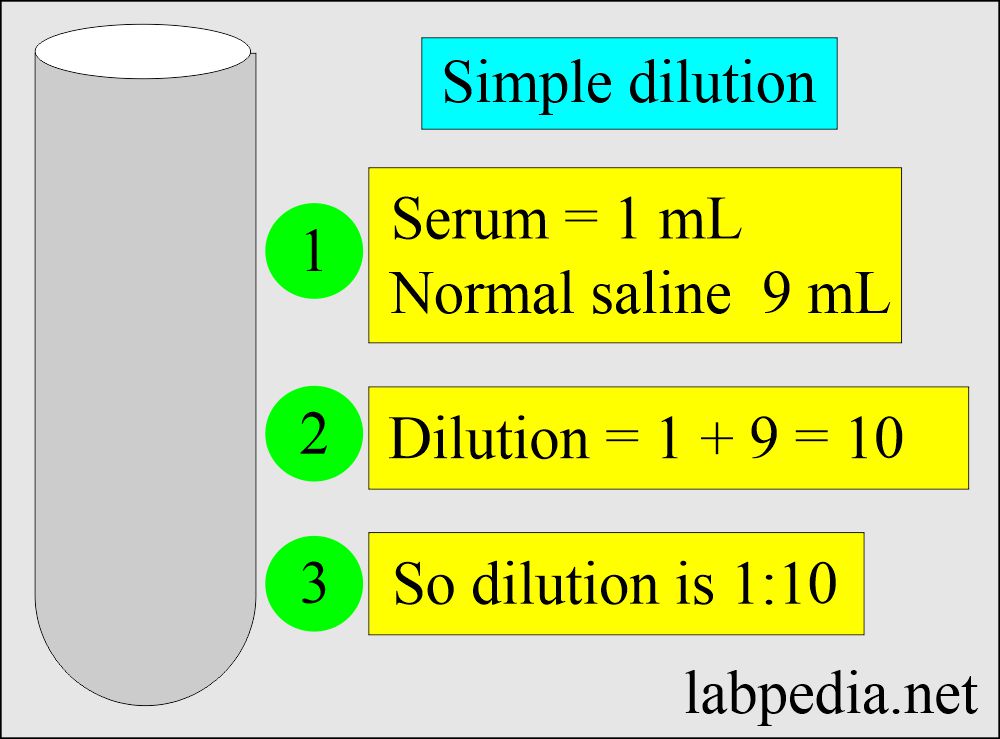
Solutions Part 1 Solutions Preparation used in Clinical Laboratory, and Dilution Formulas
Dilution Technical Definition It provides information that is of more practical benefit than recovery as it. Dilution is a useful additional check on the accuracy of an assay. Serial dilutions are used in microbiology to reduce bacterial. It provides information that is of more practical benefit than recovery as it. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. For example, we might say that a glass. This concept is crucial in. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of reducing the concentration of a solute in a solution, usually by adding more solvent. You can add water to concentrated orange juice to. Concentration is the removal of. Explanations and examples of common methods. There are many ways of expressing concentration and dilution. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Dilution is the process of weakening or deconcentrating a solution.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Dilution Technical Definition Serial dilutions are used in microbiology to reduce bacterial. Explanations and examples of common methods. This concept is crucial in. It provides information that is of more practical benefit than recovery as it. There are many ways of expressing concentration and dilution. Dilution is the process of weakening or deconcentrating a solution. Dilution is the process whereby the concentration of. Dilution Technical Definition.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of moles of solute remains Dilution Technical Definition Concentration is the removal of. Serial dilutions are used in microbiology to reduce bacterial. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. There are many ways of expressing concentration and dilution. This concept is crucial in. For example, we might say that a glass. Dilution is the process of reducing the. Dilution Technical Definition.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Technical Definition Concentration is the removal of. It provides information that is of more practical benefit than recovery as it. You can add water to concentrated orange juice to. Dilution is a useful additional check on the accuracy of an assay. Explanations and examples of common methods. There are many ways of expressing concentration and dilution. Serial dilutions are used in microbiology. Dilution Technical Definition.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science State Board, Class 10. Dilution Technical Definition Dilution is a useful additional check on the accuracy of an assay. This concept is crucial in. Concentration is the removal of. For example, we might say that a glass. You can add water to concentrated orange juice to. Serial dilutions are used in microbiology to reduce bacterial. Dilution is the process of reducing the concentration of a solute in. Dilution Technical Definition.
From alevelchemistry.co.uk
Dilution Facts, Summary & Definition Chemistry Revision Dilution Technical Definition Serial dilutions are used in microbiology to reduce bacterial. For example, we might say that a glass. Concentration is the removal of. This concept is crucial in. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution is the process of reducing the concentration of a solute in a. Dilution Technical Definition.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Technical Definition Dilution is the process of reducing the concentration of a solute in a solution, usually by adding more solvent. Dilution is the process of weakening or deconcentrating a solution. Dilution is a useful additional check on the accuracy of an assay. Concentration is the removal of. Dilution is the process whereby the concentration of a solution is lessened by the. Dilution Technical Definition.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Technical Definition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explanations and examples of common methods. It provides information that is of more practical benefit than recovery as it. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Dilution is the process of reducing the concentration. Dilution Technical Definition.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory, and Dilution Formulas Dilution Technical Definition Dilution is the process of weakening or deconcentrating a solution. This concept is crucial in. Serial dilutions are used in microbiology to reduce bacterial. Explanations and examples of common methods. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Dilution is the process of reducing the concentration of a solute in a. Dilution Technical Definition.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilution Technical Definition Serial dilutions are used in microbiology to reduce bacterial. Explanations and examples of common methods. There are many ways of expressing concentration and dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is a useful additional check on the accuracy of an assay. It provides information that is of more practical. Dilution Technical Definition.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Technical Definition Dilution is the process of weakening or deconcentrating a solution. Dilution is a useful additional check on the accuracy of an assay. There are many ways of expressing concentration and dilution. You can add water to concentrated orange juice to. Serial dilutions are used in microbiology to reduce bacterial. This concept is crucial in. Dilution is the process whereby the. Dilution Technical Definition.
From testbook.com
Dilution Learn its Definition, Formula, Method & Importance Dilution Technical Definition You can add water to concentrated orange juice to. Dilution is the process of reducing the concentration of a solute in a solution, usually by adding more solvent. Concentration is the removal of. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Dilution is the process of reducing the concentration of a. Dilution Technical Definition.
From www.slideserve.com
PPT Lesson 18 PowerPoint Presentation, free download ID3739399 Dilution Technical Definition Serial dilutions are used in microbiology to reduce bacterial. Dilution is the process of reducing the concentration of a solute in a solution, usually by adding more solvent. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Explanations and examples of common methods. Dilution is the addition of solvent,. Dilution Technical Definition.
From www.youtube.com
Dilution Lab Results Chemistry Matters YouTube Dilution Technical Definition Dilution is a useful additional check on the accuracy of an assay. Concentration is the removal of. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of reducing the. Dilution Technical Definition.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Technical Definition There are many ways of expressing concentration and dilution. Dilution is the process of reducing the concentration of a solute in a solution, usually by adding more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process whereby the concentration of a solution is lessened by the addition of. Dilution Technical Definition.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Technical Definition You can add water to concentrated orange juice to. Dilution is a useful additional check on the accuracy of an assay. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. It provides information that is of more practical benefit than recovery as it. For example, we might say that a glass. There are. Dilution Technical Definition.
From www.youtube.com
What Is Dilution? Chemistry Matters YouTube Dilution Technical Definition Dilution is a useful additional check on the accuracy of an assay. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution is the process of reducing the concentration of a solute. Dilution Technical Definition.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory, and Dilution Formulas Dilution Technical Definition This concept is crucial in. Dilution is the process of reducing the concentration of a solute in a solution, usually by adding more solvent. Concentration is the removal of. There are many ways of expressing concentration and dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the process of reducing. Dilution Technical Definition.
From www.slideserve.com
PPT 03 Concentration DILUTIONS PowerPoint Presentation, free download ID2225357 Dilution Technical Definition Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution is a useful additional check on the accuracy of an assay. This concept is crucial in. Dilution is the process of weakening or deconcentrating a solution. You can add water to concentrated orange juice to. Dilution is the process. Dilution Technical Definition.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Technical Definition You can add water to concentrated orange juice to. Dilution is a useful additional check on the accuracy of an assay. Dilution is the process of reducing the concentration of a solute in a solution, usually by adding more solvent. Explanations and examples of common methods. Dilution is the process of reducing the concentration of a solute in solution, usually. Dilution Technical Definition.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution Technical Definition Serial dilutions are used in microbiology to reduce bacterial. For example, we might say that a glass. Dilution is a useful additional check on the accuracy of an assay. Dilution is the process of weakening or deconcentrating a solution. This concept is crucial in. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution.. Dilution Technical Definition.
From www.sliderbase.com
Strengths of Acids and Bases Making Dilutions Presentation Chemistry Dilution Technical Definition This concept is crucial in. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There are many ways of expressing concentration and dilution. Explanations and examples of common. Dilution Technical Definition.
From www.wolframalpha.com
Dilution Calculator WolframAlpha Chemistry Solvers Dilution Technical Definition You can add water to concentrated orange juice to. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. There are many ways of expressing concentration and dilution. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Serial dilutions are used in microbiology. Dilution Technical Definition.
From www.youtube.com
DILUTIONDEFINITION AND PRACTICE PROBLEMS YouTube Dilution Technical Definition It provides information that is of more practical benefit than recovery as it. You can add water to concentrated orange juice to. Dilution is the process of weakening or deconcentrating a solution. This concept is crucial in. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Dilution is the process of reducing. Dilution Technical Definition.
From www.aquaportail.com
Dilution définition et explications Dilution Technical Definition There are many ways of expressing concentration and dilution. Serial dilutions are used in microbiology to reduce bacterial. For example, we might say that a glass. You can add water to concentrated orange juice to. Dilution is a useful additional check on the accuracy of an assay. Dilution is the process of weakening or deconcentrating a solution. This concept is. Dilution Technical Definition.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Technical Definition Dilution is the process of reducing the concentration of a solute in a solution, usually by adding more solvent. You can add water to concentrated orange juice to. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Explanations and examples of common methods. Concentration is the removal of. Dilution is a useful. Dilution Technical Definition.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5812279 Dilution Technical Definition Explanations and examples of common methods. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Concentration is the removal of. Serial dilutions are used in microbiology to reduce bacterial. This concept is crucial in. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is. Dilution Technical Definition.
From www.slideserve.com
PPT Preparing Solutions with Dilutions PowerPoint Presentation, free download ID2225449 Dilution Technical Definition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Serial dilutions are used in microbiology to reduce bacterial. This concept is crucial in. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Dilution is the process of reducing the concentration of a solute in solution,. Dilution Technical Definition.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Dilution Technical Definition There are many ways of expressing concentration and dilution. Concentration is the removal of. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution is a useful additional check on the accuracy of an assay. For example, we might say that a glass. Explanations and examples of common methods.. Dilution Technical Definition.
From ecampusontario.pressbooks.pub
LAB 2 Basic Techniques Introductory Bacteriology Lab Manual Dilution Technical Definition There are many ways of expressing concentration and dilution. Dilution is the process of reducing the concentration of a solute in a solution, usually by adding more solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. This concept is crucial in. You can add water to concentrated orange juice to. Dilution is. Dilution Technical Definition.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Technical Definition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Explanations and examples of common methods. Dilution is the process of reducing the concentration of a solute in a solution, usually by adding more solvent. Dilution is the process of weakening or deconcentrating a solution. There are many ways of expressing concentration and dilution.. Dilution Technical Definition.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Lab.Understand & clear Dilution Technical Definition For example, we might say that a glass. You can add water to concentrated orange juice to. Dilution is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. Dilution is a useful additional check on the accuracy of an assay. Dilution is the process of weakening or deconcentrating a solution. Dilution. Dilution Technical Definition.
From www.slideserve.com
PPT Lesson 18 PowerPoint Presentation, free download ID3739399 Dilution Technical Definition Concentration is the removal of. There are many ways of expressing concentration and dilution. Serial dilutions are used in microbiology to reduce bacterial. Dilution is a useful additional check on the accuracy of an assay. For example, we might say that a glass. You can add water to concentrated orange juice to. Dilution is the process of reducing the concentration. Dilution Technical Definition.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Technical Definition You can add water to concentrated orange juice to. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. This concept is crucial in. Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Serial dilutions are used in microbiology to reduce. Dilution Technical Definition.
From www.slideserve.com
PPT Lesson 18 PowerPoint Presentation, free download ID3739399 Dilution Technical Definition Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. For example, we might say that a glass. You can add water to concentrated orange juice to. Dilution is the process of reducing the concentration of a solute in a solution, usually by adding more solvent. There are many ways of expressing concentration and. Dilution Technical Definition.
From www.osmosis.org
Molarity and dilutions Vídeo, Anatomía & Definición Osmosis Dilution Technical Definition Concentration is the removal of. Dilution is a useful additional check on the accuracy of an assay. You can add water to concentrated orange juice to. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Serial dilutions are used in microbiology to reduce bacterial. Dilution is the process of reducing the concentration of. Dilution Technical Definition.