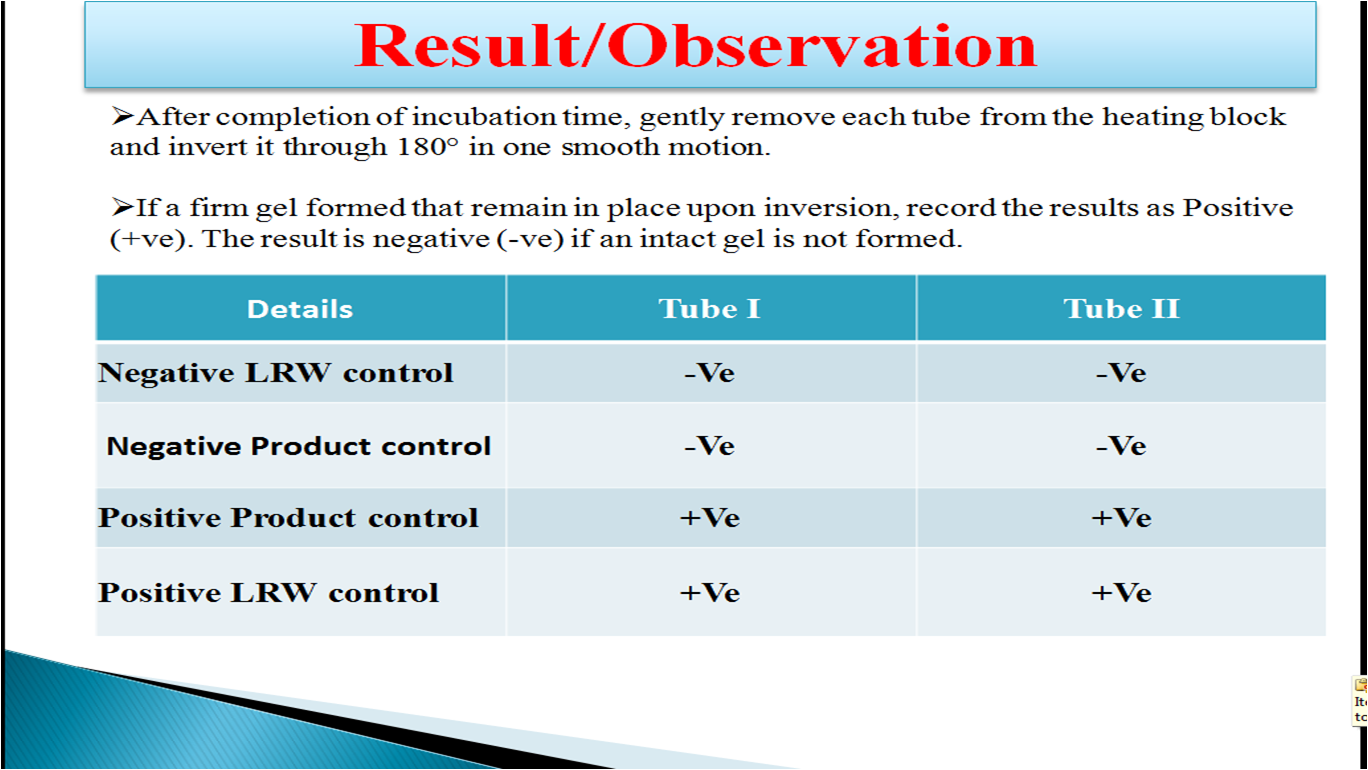
Bacterial Endotoxin Test Dilution . prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. the ph of the test dilution should be 6.0 to 8.0. when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the.
from microbiologyguideritesh.com
prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. the ph of the test dilution should be 6.0 to 8.0. finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the. we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc.
Bacterial Endotoxin Test
Bacterial Endotoxin Test Dilution the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the. when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. the ph of the test dilution should be 6.0 to 8.0. the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the. we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for.
From www.americanpharmaceuticalreview.com
Method Changes for Bacterial Endotoxins Testing (BET) Steps to Follow for a Straightforward Bacterial Endotoxin Test Dilution the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the. the ph of the test dilution should be 6.0 to 8.0. we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. prepare standard endotoxin stock solution by dissolving. Bacterial Endotoxin Test Dilution.
From www.scribd.com
Comprehensive Guide to Bacterial Endotoxin Testing Principles, Procedures, and Interpretation Bacterial Endotoxin Test Dilution finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. the ph of the test dilution should be 6.0 to 8.0. prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. we demonstrate a technique for sample preparation in which the drug substance is dissolved. Bacterial Endotoxin Test Dilution.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Bacterial Endotoxin Test Dilution prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. the ph of the test dilution should be 6.0 to 8.0. finished medical devices may also be pooled into a composite sample and. Bacterial Endotoxin Test Dilution.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Bacterial Endotoxin Test Dilution finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the. the ph of the test. Bacterial Endotoxin Test Dilution.
From www.slideshare.net
Bacterial Endotoxins test Bacterial Endotoxin Test Dilution when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and. Bacterial Endotoxin Test Dilution.
From www.slideshare.net
Bacterial Endotoxins test Bacterial Endotoxin Test Dilution finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. the ph of the test dilution should be 6.0 to 8.0. the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the. when performing the inhibition/enhancement screen, it is important to. Bacterial Endotoxin Test Dilution.
From www.scribd.com
Bacterial Endotoxin Test PDF Assay Concentration Bacterial Endotoxin Test Dilution finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. the significant changes are (i) after dilution. Bacterial Endotoxin Test Dilution.
From www.slideshare.net
Bacterial Endotoxins test Bacterial Endotoxin Test Dilution we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and. Bacterial Endotoxin Test Dilution.
From www.frontiersin.org
Frontiers Horseshoe Crab Aquaculture as a Sustainable Endotoxin Testing Source Bacterial Endotoxin Test Dilution we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. the ph of the test dilution should be 6.0 to 8.0. finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. the significant changes are (i) after dilution of endotoxin through a. Bacterial Endotoxin Test Dilution.
From www.youtube.com
USP CHAPTER 1085, “GUIDELINES ON THE ENDOTOXINS TEST” YouTube Bacterial Endotoxin Test Dilution when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. the significant changes are (i). Bacterial Endotoxin Test Dilution.
From www.scribd.com
bacterial endotoxin test Bacterial Endotoxin Test Dilution we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. the significant. Bacterial Endotoxin Test Dilution.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Bacterial Endotoxin Test Dilution the ph of the test dilution should be 6.0 to 8.0. when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. finished medical devices may also be pooled into a composite. Bacterial Endotoxin Test Dilution.
From www.scribd.com
Bacterial Endotoxin Test 14 03 17 PDF PDF Sensitivity And Specificity Lipopolysaccharide Bacterial Endotoxin Test Dilution the ph of the test dilution should be 6.0 to 8.0. the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the. when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. prepare standard endotoxin stock solution. Bacterial Endotoxin Test Dilution.
From www.slideshare.net
Bacterial Endotoxins test Bacterial Endotoxin Test Dilution the ph of the test dilution should be 6.0 to 8.0. finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the. prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia. Bacterial Endotoxin Test Dilution.
From slidetodoc.com
General Microbiology Laboratory The Serial Dilution Method of Bacterial Endotoxin Test Dilution we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and. Bacterial Endotoxin Test Dilution.
From pharmajia.com
SOP for Bacterial endotoxin test by gel clot method PharmaJia Bacterial Endotoxin Test Dilution prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. the ph of the test dilution should be 6.0 to 8.0. we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. the significant changes are (i) after dilution of endotoxin through a parallel. Bacterial Endotoxin Test Dilution.
From www.youtube.com
BET BACTERIAL ENDOTOXIN TEST YouTube Bacterial Endotoxin Test Dilution the ph of the test dilution should be 6.0 to 8.0. we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the. finished medical devices may also be pooled. Bacterial Endotoxin Test Dilution.
From www.youtube.com
Bacterial Endotoxin Testing in Compliance with ISO 117373 Testing Standard STERIS AST Bacterial Endotoxin Test Dilution the ph of the test dilution should be 6.0 to 8.0. when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. we demonstrate a technique for sample preparation in which the. Bacterial Endotoxin Test Dilution.
From www.nelsonlabs.com
Bacterial Endotoxins Test (BET) Services Nelson Labs Bacterial Endotoxin Test Dilution when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. the ph of the test dilution should be 6.0 to 8.0. we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. the significant changes are (i) after dilution. Bacterial Endotoxin Test Dilution.
From www.yumpu.com
〈85〉 BACTERIAL ENDOTOXINS TEST Bacterial Endotoxin Test Dilution the ph of the test dilution should be 6.0 to 8.0. finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard. Bacterial Endotoxin Test Dilution.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Bacterial Endotoxin Test Dilution the ph of the test dilution should be 6.0 to 8.0. the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the. we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. when performing the inhibition/enhancement screen, it is. Bacterial Endotoxin Test Dilution.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Bacterial Endotoxin Test Dilution the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the. the ph of the test dilution should be 6.0 to 8.0. when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. prepare standard endotoxin stock solution. Bacterial Endotoxin Test Dilution.
From microbeonline.com
Serial Dilution Method for Estimating Viable Count of Bacteria • Microbe Online Bacterial Endotoxin Test Dilution finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. the ph of the test dilution should be 6.0 to 8.0. the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the. when performing the inhibition/enhancement screen, it is important to. Bacterial Endotoxin Test Dilution.
From eagleanalytical.com
Bacterial Endotoxin Test USP 85 Eagle Lab Testing and Consulting for 503A and 503B Compounding Bacterial Endotoxin Test Dilution prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the. the ph of the test dilution should be 6.0 to 8.0. we demonstrate a technique for sample preparation in which the. Bacterial Endotoxin Test Dilution.
From netpharmalab.es
Bacterial endotoxins testing for safe and effective products Nerpharmalab Bacterial Endotoxin Test Dilution finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. the significant. Bacterial Endotoxin Test Dilution.
From www.researchgate.net
Serial dilution for endotoxin standards The amount of water needed to... Download Scientific Bacterial Endotoxin Test Dilution the ph of the test dilution should be 6.0 to 8.0. finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. the significant changes are (i) after dilution of endotoxin through a. Bacterial Endotoxin Test Dilution.
From www.rockland.com
Endotoxin Testing Rockland Bacterial Endotoxin Test Dilution prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. the ph of the test dilution should be 6.0 to 8.0. we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. finished medical devices may also be pooled into a composite sample and. Bacterial Endotoxin Test Dilution.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Bacterial Endotoxin Test Dilution prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. the ph of the test dilution should be 6.0 to 8.0. when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. the significant changes are (i) after dilution of endotoxin through. Bacterial Endotoxin Test Dilution.
From www.feednamer.com
WHAT ENTAILS BACTERIAL ENDOTOXIN TESTING? Feednamer Bacterial Endotoxin Test Dilution the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the. prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. the ph of the test dilution should be 6.0 to 8.0. finished medical devices may also be pooled into a composite. Bacterial Endotoxin Test Dilution.
From www.europeanpharmaceuticalreview.com
ISO publishes standard on testing bacterial endotoxins Bacterial Endotoxin Test Dilution when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. the ph of the test dilution should be 6.0 to 8.0. prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. the significant changes are (i) after dilution of endotoxin through. Bacterial Endotoxin Test Dilution.
From www.scribd.com
85 Bacterial Endotoxins Test Change To Read PDF Scientific Control Ph Bacterial Endotoxin Test Dilution prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the. when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. we. Bacterial Endotoxin Test Dilution.
From pharmadekho.com
Bacterial endotoxin test for dexamethasone injection Pharma Dekho Bacterial Endotoxin Test Dilution prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. the ph of the test. Bacterial Endotoxin Test Dilution.
From www.europeanpharmaceuticalreview.com
Bacterial Endotoxin Test using LAL interfering factors Bacterial Endotoxin Test Dilution the ph of the test dilution should be 6.0 to 8.0. finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. the significant changes are (i) after dilution of endotoxin through a. Bacterial Endotoxin Test Dilution.
From zhuanlan.zhihu.com
Bacterial Endotix Testing (BET) 细菌内毒素测试 知乎 Bacterial Endotoxin Test Dilution prepare standard endotoxin stock solution by dissolving japanese pharmacopoeia reference standard endotoxin in water for. when performing the inhibition/enhancement screen, it is important to first calculate the endotoxin limit and the mvd or the mvc. the ph of the test dilution should be 6.0 to 8.0. the significant changes are (i) after dilution of endotoxin through. Bacterial Endotoxin Test Dilution.
From www.researchgate.net
Endotoxin limit (EL) and maximum valid dilution (MVD) of matrices Download Scientific Diagram Bacterial Endotoxin Test Dilution we demonstrate a technique for sample preparation in which the drug substance is dissolved in dimethyl sulfoxide (dmso),. the significant changes are (i) after dilution of endotoxin through a parallel set of solutions, one containing water and the. finished medical devices may also be pooled into a composite sample and assayed for bacterial endotoxins. prepare standard. Bacterial Endotoxin Test Dilution.