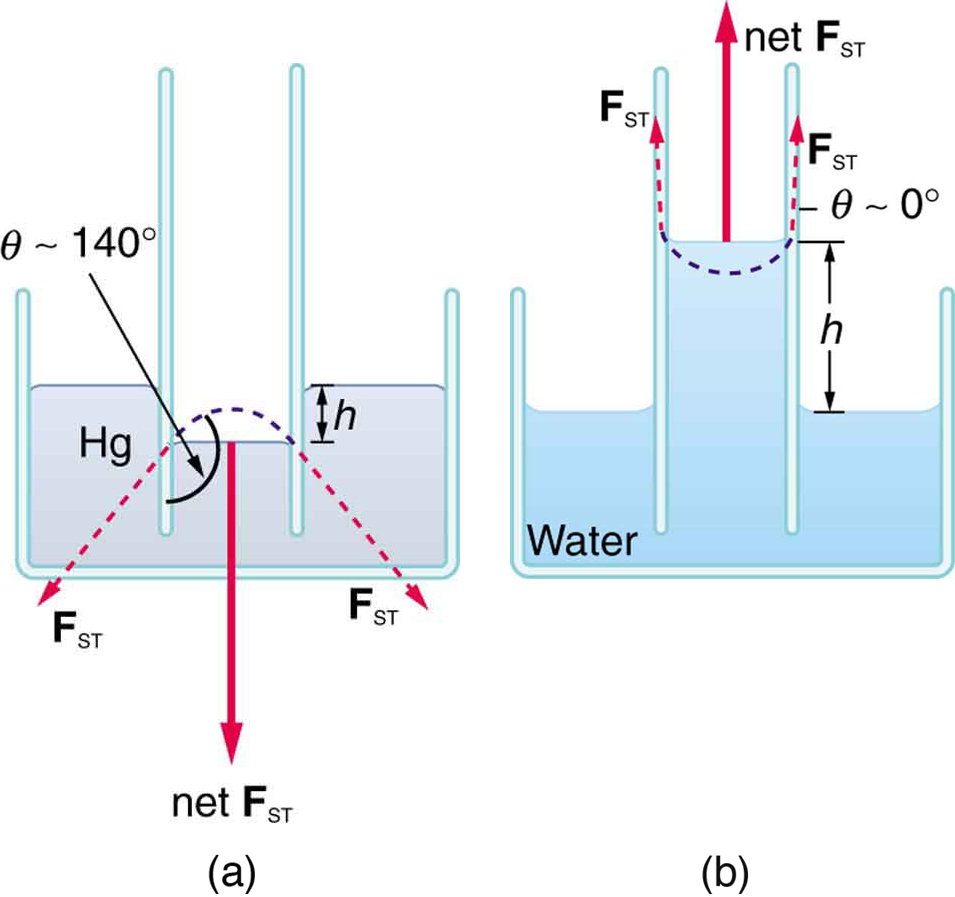
Capillary Tubes Meniscus . When water is confined in a glass tube, its meniscus (surface) has a concave shape because the water wets the glass and creeps up the side of the tube. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section. The contact angle is determined. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules.
from pressbooks.online.ucf.edu
The contact angle is determined. When water is confined in a glass tube, its meniscus (surface) has a concave shape because the water wets the glass and creeps up the side of the tube. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section.
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary
Capillary Tubes Meniscus Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. When water is confined in a glass tube, its meniscus (surface) has a concave shape because the water wets the glass and creeps up the side of the tube. The contact angle is determined. The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section.
From www.alamy.com
Capillary action tube Stock Vector Images Alamy Capillary Tubes Meniscus Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. The contact angle is determined. On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. When water is confined in a glass tube, its meniscus (surface) has a concave shape. Capillary Tubes Meniscus.
From www.researchgate.net
(a) Sketch of capillary rise in a vertical tube. (b) Sketch of the Capillary Tubes Meniscus Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. The contact angle is determined. The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section. On the other hand, the cohesive forces. Capillary Tubes Meniscus.
From www.mdpi.com
Computation Free FullText Contact Angle Effects on Pore and Corner Capillary Tubes Meniscus The contact angle is determined. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. When water is confined in a glass tube, its meniscus (surface) has a concave shape because the water wets the glass and creeps up the side of the tube. Alternatively for mercury, the cohesive forces are. Capillary Tubes Meniscus.
From www.alamy.com
Capillary action tube Stock Vector Images Alamy Capillary Tubes Meniscus The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. The contact angle is determined. On the other hand, the cohesive forces. Capillary Tubes Meniscus.
From physics.stackexchange.com
fluid statics Why is there excess pressure on the concave side of Capillary Tubes Meniscus The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section. On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. The contact angle is determined. Capillary action occurs when the adhesion to the walls is stronger than the. Capillary Tubes Meniscus.
From physicsnotebook.com
What Is The Shape Of A Liquid Meniscus In A Capillary Tube? Physics Capillary Tubes Meniscus On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. The contact angle is determined. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. When water is confined in a glass. Capillary Tubes Meniscus.
From www.numerade.com
SOLVED O.lmm Capillary Convex meniscus Hg bulk surface level lllmm Capillary Tubes Meniscus Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. When water is confined in a glass tube, its meniscus (surface) has a concave shape because the water wets the glass and creeps up the side of the tube. On the other hand,. Capillary Tubes Meniscus.
From www.quirkyscience.com
Capillary Action from the Forces of Adhesion and Cohesion Capillary Tubes Meniscus Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section. On the other hand, the cohesive forces between mercury atoms are much. Capillary Tubes Meniscus.
From www.researchgate.net
(a) Meniscus shape for various wettability capillary tube at Capillary Tubes Meniscus On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away. Capillary Tubes Meniscus.
From www.researchgate.net
Mechanism of inner microstructures enhancing capillary rise in tubes Capillary Tubes Meniscus On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. When water is confined in a glass tube, its meniscus (surface) has a concave shape because the water wets the. Capillary Tubes Meniscus.
From www.davidlnelson.md
Trees & Capillary Action Capillary Tubes Meniscus On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section. When water is confined in a glass tube, its meniscus (surface) has a concave shape because the water. Capillary Tubes Meniscus.
From www.researchgate.net
Permeablesurface situation for capillary imbibition where a meniscus Capillary Tubes Meniscus Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. When water is confined in a glass tube, its meniscus (surface) has a concave shape because the water wets the glass and creeps up the side of the tube. On the other hand, the cohesive forces between mercury atoms are much. Capillary Tubes Meniscus.
From www.earthslab.com
Medial Meniscus Earth's Lab Capillary Tubes Meniscus The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section. On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. The contact angle is determined. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which. Capillary Tubes Meniscus.
From www.alamy.com
Capillary action tube Stock Vector Images Alamy Capillary Tubes Meniscus When water is confined in a glass tube, its meniscus (surface) has a concave shape because the water wets the glass and creeps up the side of the tube. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces. Capillary Tubes Meniscus.
From www.semanticscholar.org
Figure 2 from HYDRODYNAMICS OF A CONFINED MENISCUS IN A SQUARE Capillary Tubes Meniscus Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section. On the other hand, the cohesive forces between mercury atoms are much. Capillary Tubes Meniscus.
From mavink.com
Capillary Tube Diagram Capillary Tubes Meniscus Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. The contact angle is determined. On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the. Capillary Tubes Meniscus.
From www.academia.edu
(PDF) Modeling meniscus rise in capillary tubes using fluid in rigid Capillary Tubes Meniscus Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section. The contact angle is determined. When water is confined in a glass. Capillary Tubes Meniscus.
From www.chegg.com
Solved A Liquid In A Capillary Tube Forms A Meniscus That... Capillary Tubes Meniscus On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. When water is confined in a glass tube, its meniscus (surface) has a concave shape because the water wets the. Capillary Tubes Meniscus.
From sciencenotes.org
Adhesion vs Cohesion Capillary Tubes Meniscus Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section. On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and. Capillary Tubes Meniscus.
From www.researchgate.net
Simulated meniscus height in coated capillary tubes of different radii Capillary Tubes Meniscus The contact angle is determined. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. On the other hand, the cohesive forces between mercury. Capillary Tubes Meniscus.
From www.researchgate.net
Capillary meniscus model and microscopic phase interface movement Capillary Tubes Meniscus The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section. The contact angle is determined. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. Capillary action occurs when the adhesion to. Capillary Tubes Meniscus.
From www.animalia-life.club
Adhesion Of Water Meniscus Capillary Tubes Meniscus When water is confined in a glass tube, its meniscus (surface) has a concave shape because the water wets the glass and creeps up the side of the tube. The contact angle is determined. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. Alternatively for mercury, the cohesive forces are. Capillary Tubes Meniscus.
From byjus.com
what happens when the calculated value of h in capillary action exceeds Capillary Tubes Meniscus The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section. The contact angle is determined. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the. Capillary Tubes Meniscus.
From www.tutorix.com
To find the Surface Tension of Water by Capillary Rise Method Capillary Tubes Meniscus The contact angle is determined. When water is confined in a glass tube, its meniscus (surface) has a concave shape because the water wets the glass and creeps up the side of the tube. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. Alternatively for mercury, the cohesive forces are. Capillary Tubes Meniscus.
From pressbooks.online.ucf.edu
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary Capillary Tubes Meniscus On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. Capillary action occurs when the adhesion to the walls is stronger than. Capillary Tubes Meniscus.
From www.toppr.com
Figure shows a capillary tube of radius r dipped into water. If the Capillary Tubes Meniscus On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid. Capillary Tubes Meniscus.
From www.slideserve.com
PPT Lecture 15 Capillary motion PowerPoint Presentation, free Capillary Tubes Meniscus Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. The meniscus forms an angle θ, the contact angle, with the inner wall of. Capillary Tubes Meniscus.
From www.researchgate.net
Schematic of the meniscus region in the capillary slot Download Capillary Tubes Meniscus On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. When water is confined in a glass tube, its meniscus (surface) has a concave shape because the water wets the. Capillary Tubes Meniscus.
From www.quirkyscience.com
Capillary Action from the Forces of Adhesion and Cohesion Capillary Tubes Meniscus Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. When water is confined in a glass tube, its meniscus (surface) has a concave shape because the water wets the. Capillary Tubes Meniscus.
From www.researchgate.net
Space coordinates and meniscus in the capillary tube Download Capillary Tubes Meniscus Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section. When water is confined in a glass tube, its meniscus (surface) has. Capillary Tubes Meniscus.
From www.normasabnt.org
Ação Capilar (capilaridade) o que significa, como funciona, exemplos Capillary Tubes Meniscus On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. The contact angle is determined. Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. Capillary action occurs when the adhesion to. Capillary Tubes Meniscus.
From sciencenotes.org
Capillary Action What It Is and How It Works Capillary Tubes Meniscus On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. The contact angle is determined. When water is confined in a glass tube, its meniscus (surface) has a concave shape because the water wets the glass and creeps up the side of the tube. Alternatively for mercury, the cohesive. Capillary Tubes Meniscus.
From www.youtube.com
contact angleshape of liquid meniscuscapillarityrise of liquid in Capillary Tubes Meniscus On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. The contact angle is determined. The meniscus forms an angle θ, the contact angle, with the inner wall of a capillary tube with circular cross section. Capillary action occurs when the adhesion to the walls is stronger than the. Capillary Tubes Meniscus.
From joelgordon.photoshelter.com
Meniscus Joel Gordon Photography Capillary Tubes Meniscus On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. When water is confined in a glass tube, its meniscus (surface) has a concave shape because the water wets the glass and creeps up the side of the tube. Alternatively for mercury, the cohesive forces are stronger than the. Capillary Tubes Meniscus.
From www.pdfprof.com
degree sum formula proof Capillary Tubes Meniscus Alternatively for mercury, the cohesive forces are stronger than the adhesive forces which allows the the meniscus to bend away from the walls of the capillary tube. On the other hand, the cohesive forces between mercury atoms are much greater than the adhesive forces between mercury and glass. Capillary action occurs when the adhesion to the walls is stronger than. Capillary Tubes Meniscus.