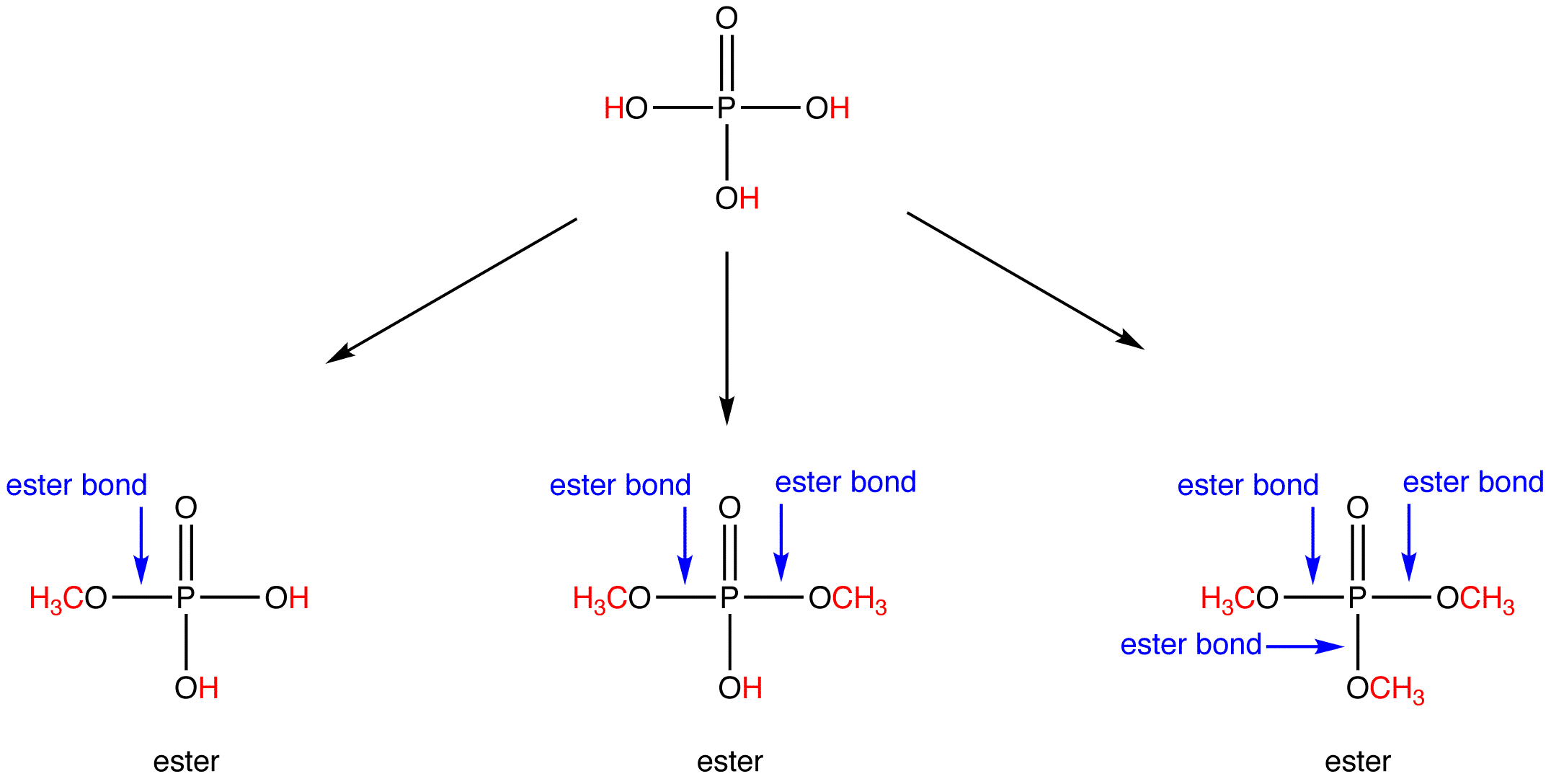
What Is The R In An Ester . Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. An ester bond is a linkage. Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups), are commonly prepared by reaction of carboxylic acids and alcohols in the presence of hydrochloric acid. The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic acid, and r′ is the alcohol. The r group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group. The alkyl fragments gain mgbr to form a grignard reagent. What is an ester bond? The r groups denote the rest of the molecule not included in the functional group.
from chem.libretexts.org
The alkyl fragments gain mgbr to form a grignard reagent. An ester bond is a linkage. The r groups denote the rest of the molecule not included in the functional group. The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic acid, and r′ is the alcohol. Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. The r group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group. Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups), are commonly prepared by reaction of carboxylic acids and alcohols in the presence of hydrochloric acid. What is an ester bond?
Ester Chemistry LibreTexts
What Is The R In An Ester Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. What is an ester bond? The r groups denote the rest of the molecule not included in the functional group. The alkyl fragments gain mgbr to form a grignard reagent. An ester bond is a linkage. Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic acid, and r′ is the alcohol. Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups), are commonly prepared by reaction of carboxylic acids and alcohols in the presence of hydrochloric acid. The r group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group.
From www.masterorganicchemistry.com
Fischer Esterification Carboxylic Acid to Ester Under Acidic What Is The R In An Ester What is an ester bond? Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups), are commonly prepared by reaction of carboxylic acids and alcohols in the presence of hydrochloric acid. The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic acid, and r′ is the. What Is The R In An Ester.
From www.slideserve.com
PPT What are esters? PowerPoint Presentation, free download ID6693863 What Is The R In An Ester An ester bond is a linkage. The r group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group. The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic acid, and r′ is the alcohol. The alkyl fragments gain mgbr to. What Is The R In An Ester.
From www.animalia-life.club
Ester Functional Group Examples What Is The R In An Ester Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups), are commonly prepared by reaction of carboxylic acids and alcohols in the presence of hydrochloric acid. The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic acid, and r′ is the alcohol. The r group of. What Is The R In An Ester.
From brainly.com
Which functional group is found in an ester? a central carbon is double What Is The R In An Ester An ester bond is a linkage. What is an ester bond? Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. The alkyl fragments gain mgbr to form a grignard reagent. The r group of the ester is largely unimportant to the overall. What Is The R In An Ester.
From www.thoughtco.com
What Is an Ester in Chemistry? What Is The R In An Ester The r groups denote the rest of the molecule not included in the functional group. Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups), are commonly prepared by reaction of carboxylic acids and alcohols in the presence of hydrochloric acid. What is an ester bond? An ester bond is a linkage. The r group of the. What Is The R In An Ester.
From chem.libretexts.org
Ester Chemistry LibreTexts What Is The R In An Ester What is an ester bond? The r group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group. The r groups denote the rest of the molecule not included in the functional group. The alkyl fragments gain mgbr to form a grignard reagent. Esters have the general formula,, where r may be. What Is The R In An Ester.
From www.chegg.com
Solved 1) Identify R and R' in the equation above when the What Is The R In An Ester The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic acid, and r′ is the alcohol. The alkyl fragments gain mgbr to form a grignard reagent. Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be. What Is The R In An Ester.
From www.online-sciences.com
Physical and chemical properties of Esters, Esterification reaction and What Is The R In An Ester Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. What is an ester bond? The r group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group. The alkyl fragments gain mgbr to form. What Is The R In An Ester.
From byjus.com
What is a general formula for an ester? What Is The R In An Ester What is an ester bond? The r group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group. An ester bond is a linkage. Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups), are commonly prepared by reaction of carboxylic acids and alcohols in the presence of hydrochloric. What Is The R In An Ester.
From www.chemistrysteps.com
Fischer Esterification Chemistry Steps What Is The R In An Ester Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic acid, and r′ is the alcohol. An ester bond is a linkage.. What Is The R In An Ester.
From www.youtube.com
General formula of ester YouTube What Is The R In An Ester The r groups denote the rest of the molecule not included in the functional group. What is an ester bond? Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups), are commonly prepared by reaction of carboxylic acids and alcohols in the presence of hydrochloric acid. An ester bond is a linkage. The alkyl fragments gain mgbr. What Is The R In An Ester.
From www.slideserve.com
PPT The Major Functional Groups PowerPoint Presentation, free What Is The R In An Ester Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups), are commonly prepared by reaction of carboxylic acids and alcohols in the presence of hydrochloric acid. The alkyl fragments gain mgbr to form a grignard reagent. The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic. What Is The R In An Ester.
From www.youtube.com
What are Esters? Structure, Nomenclature, Boiling and Solubility of What Is The R In An Ester An ester bond is a linkage. The r group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group. Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. The alkyl fragments gain mgbr to. What Is The R In An Ester.
From byjus.com
Difference Between Ester and Ether Ester vs Ether BYJU'S What Is The R In An Ester The alkyl fragments gain mgbr to form a grignard reagent. The r groups denote the rest of the molecule not included in the functional group. The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic acid, and r′ is the alcohol. What is an ester bond? The r group. What Is The R In An Ester.
From www.animalia-life.club
Esterification Mechanism What Is The R In An Ester The alkyl fragments gain mgbr to form a grignard reagent. The r group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group. The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic acid, and r′ is the alcohol. Carboxylic acid. What Is The R In An Ester.
From www.animalia-life.club
Ester Functional Group Examples What Is The R In An Ester The alkyl fragments gain mgbr to form a grignard reagent. What is an ester bond? An ester bond is a linkage. The r group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group. The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts. What Is The R In An Ester.
From www.masterorganicchemistry.com
Conversion of carboxylic acids to esters using acid and alcohols What Is The R In An Ester What is an ester bond? Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups), are commonly prepared by reaction of carboxylic acids and alcohols in the presence of hydrochloric acid. The alkyl fragments gain mgbr to form a grignard reagent. The r group of the ester is largely unimportant to the overall reaction and is typically. What Is The R In An Ester.
From www.slideserve.com
PPT Creating an Ester PowerPoint Presentation, free download ID4674872 What Is The R In An Ester The alkyl fragments gain mgbr to form a grignard reagent. What is an ester bond? An ester bond is a linkage. Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining. What Is The R In An Ester.
From www.reddit.com
Chemistry Teacher AMA r/GCSE What Is The R In An Ester The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic acid, and r′ is the alcohol. The r group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group. An ester bond is a linkage. Esters have the general formula,, where. What Is The R In An Ester.
From www.chemistrysteps.com
Naming Esters Chemistry Steps What Is The R In An Ester The r group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group. Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups), are commonly prepared by reaction of carboxylic acids and alcohols in the presence of hydrochloric acid. Esters have the general formula,, where r may be a. What Is The R In An Ester.
From ar.inspiredpencil.com
Naming Esters What Is The R In An Ester The r group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group. The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic acid, and r′ is the alcohol. Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining. What Is The R In An Ester.
From what-is-this.net
ester définition What is What Is The R In An Ester An ester bond is a linkage. The alkyl fragments gain mgbr to form a grignard reagent. What is an ester bond? The r groups denote the rest of the molecule not included in the functional group. The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic acid, and r′. What Is The R In An Ester.
From chemistnotes.com
Esters Definition, Nomenclature, Preparation, Properties, and What Is The R In An Ester The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic acid, and r′ is the alcohol. An ester bond is a linkage. Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups), are commonly prepared by reaction of carboxylic acids and alcohols in the presence of. What Is The R In An Ester.
From www.slideserve.com
PPT What are esters? PowerPoint Presentation, free download ID6693863 What Is The R In An Ester The r group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group. The alkyl fragments gain mgbr to form a grignard reagent. What is an ester bond? Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an. What Is The R In An Ester.
From www.chemistrysteps.com
Naming Esters Chemistry Steps What Is The R In An Ester The r group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group. An ester bond is a linkage. Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. What is an ester bond? Carboxylic. What Is The R In An Ester.
From www.chemistrysteps.com
Ester Reactions Summary and Practice Problems Chemistry Steps What Is The R In An Ester Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups), are commonly prepared by reaction of carboxylic acids and alcohols in the presence of hydrochloric acid. Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. The alkyl fragments gain. What Is The R In An Ester.
From www.youtube.com
Esterification Reaction Formation of Ester Organic Chemistry YouTube What Is The R In An Ester What is an ester bond? The r group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group. Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. Carboxylic acid esters, formula rcoor′ (r and. What Is The R In An Ester.
From www.difference101.com
Ether vs. Ester 9 Key Differences, Definition, Examples Difference 101 What Is The R In An Ester Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic acid, and r′ is the alcohol. Carboxylic acid esters, formula rcoor′ (r. What Is The R In An Ester.
From chem.libretexts.org
Ester Chemistry LibreTexts What Is The R In An Ester The r groups denote the rest of the molecule not included in the functional group. Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups), are commonly prepared by reaction of carboxylic acids and alcohols in the presence of hydrochloric acid. The alkyl fragments gain mgbr to form a grignard reagent. Esters have the general formula,, where. What Is The R In An Ester.
From www.slideserve.com
PPT Esters PowerPoint Presentation, free download ID2192073 What Is The R In An Ester Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. The r groups denote the rest of the molecule not included in the functional group. What is an ester bond? An ester bond is a linkage. The r group of the ester is. What Is The R In An Ester.
From www.masterorganicchemistry.com
Reactions of Grignard Reagents Master Organic Chemistry What Is The R In An Ester Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. The alkyl fragments gain mgbr to form a grignard reagent. What is an ester bond? The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts. What Is The R In An Ester.
From www.animalia-life.club
Ester Functional Group Examples What Is The R In An Ester Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups), are commonly prepared by reaction of carboxylic acids and alcohols in the presence of hydrochloric acid. What is an ester. What Is The R In An Ester.
From chemdictionary.org
Ester 2 • Chemistry Dictionary What Is The R In An Ester The alkyl fragments gain mgbr to form a grignard reagent. What is an ester bond? Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups), are commonly prepared by reaction of carboxylic acids and alcohols in the presence of hydrochloric acid. The chemical formula of an ester takes the form rco 2 r′, where r is the. What Is The R In An Ester.
From www.chemistrysteps.com
Esters Reaction with Amines The Aminolysis Mechanism Chemistry Steps What Is The R In An Ester The alkyl fragments gain mgbr to form a grignard reagent. What is an ester bond? Esters have the general formula,, where r may be a hydrogen atom, an alkyl group, or an aryl group, and r′ may be an alkyl group or. The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts. What Is The R In An Ester.
From www.chemistrysteps.com
Ester Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps What Is The R In An Ester The chemical formula of an ester takes the form rco 2 r′, where r is the hydrocarbon parts of the carboxylic acid, and r′ is the alcohol. The r groups denote the rest of the molecule not included in the functional group. What is an ester bond? Carboxylic acid esters, formula rcoor′ (r and r′ are any organic combining groups),. What Is The R In An Ester.