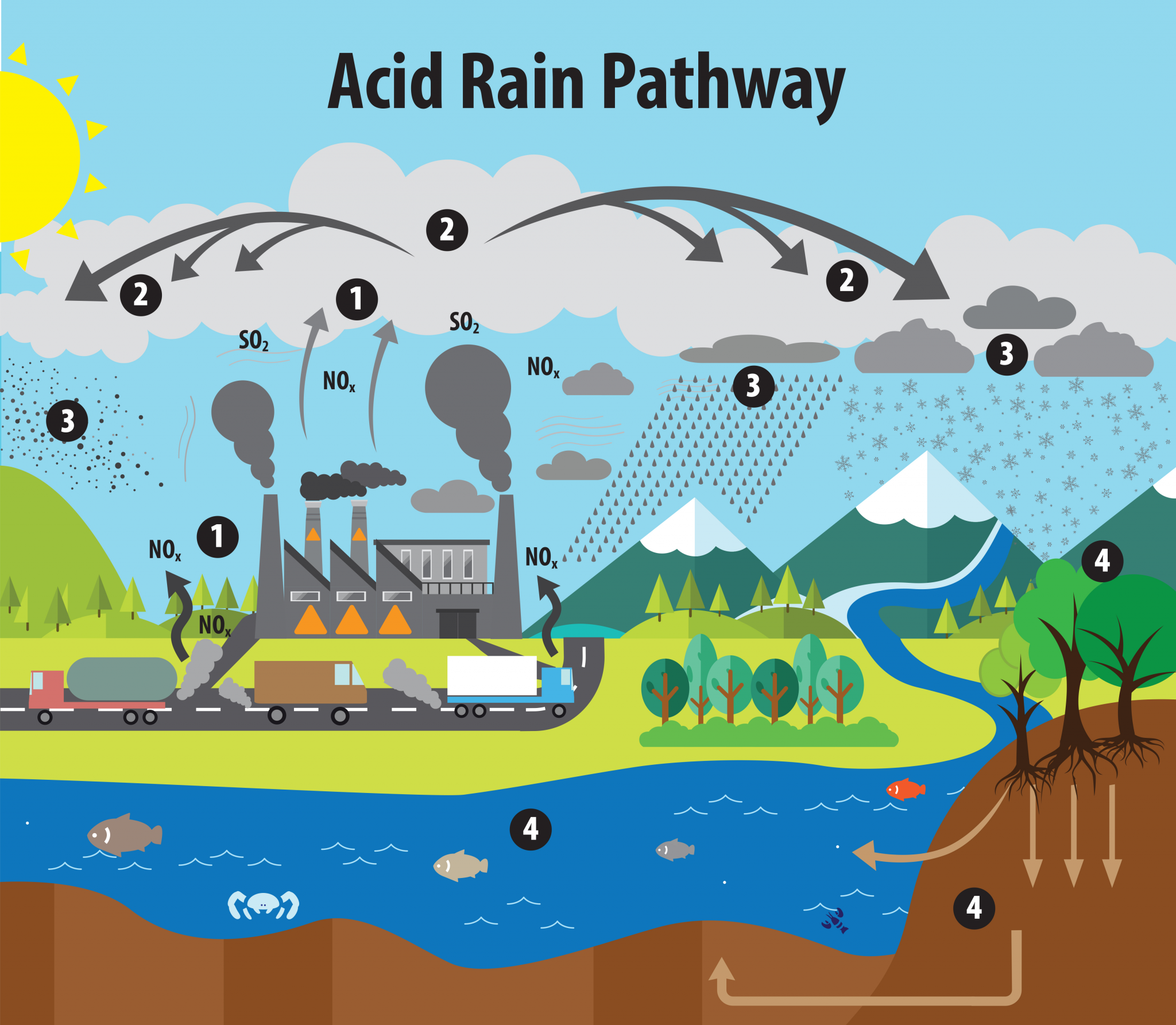
Why Does Acid Rain Weather Limestone Bedrock . When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. What happens in a reaction between acid rain and limestone? Limestone is mostly made up of the mineral calcium carbonate (caco3). As rain falls to the ground, it. Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water due to acid rain than areas with igneous bedrock.
from www.breeze-technologies.de
As rain falls to the ground, it. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. Limestone is mostly made up of the mineral calcium carbonate (caco3). Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. What happens in a reaction between acid rain and limestone? On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water due to acid rain than areas with igneous bedrock.
How air pollution causes acid rain Breeze Technologies
Why Does Acid Rain Weather Limestone Bedrock When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Limestone is mostly made up of the mineral calcium carbonate (caco3). Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. As rain falls to the ground, it. What happens in a reaction between acid rain and limestone? Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water due to acid rain than areas with igneous bedrock.
From www.biologyexams4u.com
Acid rain Definition, Causes and Effects Acid Rain Formula Why Does Acid Rain Weather Limestone Bedrock Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. Limestone is mostly made up of the mineral calcium carbonate (caco3). On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water due to acid rain than areas with. Why Does Acid Rain Weather Limestone Bedrock.
From www.dreamstime.com
Limestone Surface Eroded by Acid Rain. Stock Image Image of gray Why Does Acid Rain Weather Limestone Bedrock What happens in a reaction between acid rain and limestone? When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. As rain falls to the ground, it. Limestone is mostly made up of the mineral calcium carbonate (caco3). Chemical weathering increases steadily upward in the weathered bedrock, with. Why Does Acid Rain Weather Limestone Bedrock.
From www.climateandweather.net
Acid Rain Climate & Weather Why Does Acid Rain Weather Limestone Bedrock When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water due to acid rain than areas with igneous bedrock. As rain falls to the ground, it.. Why Does Acid Rain Weather Limestone Bedrock.
From www.thenakedscientists.com
What happens when acid reacts with limestone? Science Questions Why Does Acid Rain Weather Limestone Bedrock Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. As rain falls to the ground, it. What happens in a reaction between acid rain and limestone? Limestone. Why Does Acid Rain Weather Limestone Bedrock.
From ar.inspiredpencil.com
Acid Precipitation On Rocks Why Does Acid Rain Weather Limestone Bedrock When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. What happens in a reaction between acid rain and limestone? On the positive side, regions where the bedrock. Why Does Acid Rain Weather Limestone Bedrock.
From slidetodoc.com
Looking Back How does acid rain weather rocks Why Does Acid Rain Weather Limestone Bedrock As rain falls to the ground, it. Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. What happens in a reaction between acid rain and limestone? Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. Limestone is mostly. Why Does Acid Rain Weather Limestone Bedrock.
From lessondbharvey.z13.web.core.windows.net
Diagram Of Acid Rain Why Does Acid Rain Weather Limestone Bedrock Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. As rain falls to the ground, it. Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. Limestone is mostly made up of the mineral calcium carbonate (caco3). When sulfurous,. Why Does Acid Rain Weather Limestone Bedrock.
From ar.inspiredpencil.com
Limestone Building Acid Rain Why Does Acid Rain Weather Limestone Bedrock What happens in a reaction between acid rain and limestone? As rain falls to the ground, it. Limestone is mostly made up of the mineral calcium carbonate (caco3). On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water due to acid rain than areas with igneous bedrock. Limestone. Why Does Acid Rain Weather Limestone Bedrock.
From socratic.org
How does limestone change throughout the rock cycle? Socratic Why Does Acid Rain Weather Limestone Bedrock As rain falls to the ground, it. Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. On the positive side, regions where the bedrock or soil contain large amounts. Why Does Acid Rain Weather Limestone Bedrock.
From www.slideserve.com
PPT Weathering and Soil Formation (Chapter 6) PowerPoint Presentation Why Does Acid Rain Weather Limestone Bedrock Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. As rain falls to the ground, it. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. On the positive side, regions where the bedrock or soil contain. Why Does Acid Rain Weather Limestone Bedrock.
From www.scienceshorts.com
How Does Limestone Protect Lakes from Acid Rain Why Does Acid Rain Weather Limestone Bedrock Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. Limestone is mostly made up of the mineral calcium carbonate (caco3). As rain falls to the ground, it. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves.. Why Does Acid Rain Weather Limestone Bedrock.
From www.tes.com
Limestone Erosion and Acid Rain Teaching Resources Why Does Acid Rain Weather Limestone Bedrock Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. What happens in a reaction between acid rain and limestone? On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water due to acid rain than areas with igneous. Why Does Acid Rain Weather Limestone Bedrock.
From www.scribd.com
Effect of Acid Rain On Limestone Rock PDF Limestone Chemistry Why Does Acid Rain Weather Limestone Bedrock Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the. Why Does Acid Rain Weather Limestone Bedrock.
From softbacktravel.com
What is Acid Rain & How is it Formed? Softback Travel Why Does Acid Rain Weather Limestone Bedrock When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. What happens in a reaction between acid rain and limestone? Chemical weathering increases steadily upward in the weathered. Why Does Acid Rain Weather Limestone Bedrock.
From www.slideshare.net
034 acid rain erodes houses made of limestone Why Does Acid Rain Weather Limestone Bedrock As rain falls to the ground, it. Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. What happens in a reaction between acid rain and limestone? Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. On the positive. Why Does Acid Rain Weather Limestone Bedrock.
From www.slideserve.com
PPT Acid Rain PowerPoint Presentation ID2884717 Why Does Acid Rain Weather Limestone Bedrock Limestone is mostly made up of the mineral calcium carbonate (caco3). When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. What happens in a reaction between acid. Why Does Acid Rain Weather Limestone Bedrock.
From www.worldatlas.com
What Is Acid Rain? WorldAtlas Why Does Acid Rain Weather Limestone Bedrock Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water due to acid rain than areas with igneous bedrock. Chemical weathering increases steadily upward in the weathered bedrock,. Why Does Acid Rain Weather Limestone Bedrock.
From www.scienceshorts.com
How Does Limestone Protect Lakes from Acid Rain Why Does Acid Rain Weather Limestone Bedrock On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water due to acid rain than areas with igneous bedrock. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. What happens in a reaction between acid. Why Does Acid Rain Weather Limestone Bedrock.
From www.internetgeography.net
How does weathering affect limestone? Geography Why Does Acid Rain Weather Limestone Bedrock Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. As rain falls to the ground, it. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Limestone is mostly made up of the mineral calcium carbonate (caco3).. Why Does Acid Rain Weather Limestone Bedrock.
From ar.inspiredpencil.com
Acid Precipitation On Rocks Why Does Acid Rain Weather Limestone Bedrock When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. As rain falls to the ground, it. On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water due to acid rain than areas with igneous bedrock.. Why Does Acid Rain Weather Limestone Bedrock.
From www.scribd.com
Effect of Acid Rain On Limestone Rock PDF Limestone Sulfuric Acid Why Does Acid Rain Weather Limestone Bedrock When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. As rain falls to the ground, it. Limestone is mostly made up of the mineral calcium carbonate (caco3). Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater.. Why Does Acid Rain Weather Limestone Bedrock.
From www.breeze-technologies.de
How air pollution causes acid rain Breeze Technologies Why Does Acid Rain Weather Limestone Bedrock As rain falls to the ground, it. Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water due to acid rain than areas with igneous bedrock. Limestone is. Why Does Acid Rain Weather Limestone Bedrock.
From www.dreamstime.com
Limestone Surface Eroded by Acid Rain. Stock Photo Image of pattern Why Does Acid Rain Weather Limestone Bedrock Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. As rain falls to the ground, it. On the positive side, regions where the bedrock or soil contain. Why Does Acid Rain Weather Limestone Bedrock.
From slideplayer.com
Weathering The process in which rocks are FIRST broken down by chemical Why Does Acid Rain Weather Limestone Bedrock What happens in a reaction between acid rain and limestone? Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. Limestone is mostly made up of the mineral calcium carbonate (caco3). Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and. Why Does Acid Rain Weather Limestone Bedrock.
From www.scienceshorts.com
How Does Limestone Protect Lakes from Acid Rain Why Does Acid Rain Weather Limestone Bedrock Limestone is mostly made up of the mineral calcium carbonate (caco3). As rain falls to the ground, it. Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water. Why Does Acid Rain Weather Limestone Bedrock.
From slidetodoc.com
Looking Back How does acid rain weather rocks Why Does Acid Rain Weather Limestone Bedrock What happens in a reaction between acid rain and limestone? Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water due to acid rain than areas with igneous. Why Does Acid Rain Weather Limestone Bedrock.
From slideplayer.com
Acids, Bases, and Salts. ppt download Why Does Acid Rain Weather Limestone Bedrock Limestone is mostly made up of the mineral calcium carbonate (caco3). What happens in a reaction between acid rain and limestone? On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water due to acid rain than areas with igneous bedrock. As rain falls to the ground, it. Chemical. Why Does Acid Rain Weather Limestone Bedrock.
From www.dreamstime.com
The Texture of Limestone Rock is Eroded by Acid and Rain. Stock Image Why Does Acid Rain Weather Limestone Bedrock Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water due to acid rain than areas with igneous bedrock. Limestone caves form due to the chemical weathering of. Why Does Acid Rain Weather Limestone Bedrock.
From finwise.edu.vn
List 101+ Pictures When Acid Rain Falls On Limestone What Weathering Why Does Acid Rain Weather Limestone Bedrock Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. As rain falls to the ground, it. What happens in a reaction between acid rain and limestone? Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. When sulfurous, sulfuric,. Why Does Acid Rain Weather Limestone Bedrock.
From slidetodoc.com
Looking Back How does acid rain weather rocks Why Does Acid Rain Weather Limestone Bedrock Limestone is mostly made up of the mineral calcium carbonate (caco3). As rain falls to the ground, it. What happens in a reaction between acid rain and limestone? Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. On the positive side, regions where the bedrock or soil contain large. Why Does Acid Rain Weather Limestone Bedrock.
From www.scienceshorts.com
What Type of Rock Weathers Most Rapidly When Exposed to Acid Rain Why Does Acid Rain Weather Limestone Bedrock When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in groundwater and rainwater. As rain falls to the ground, it. Chemical weathering increases steadily upward in the weathered bedrock, with intervals. Why Does Acid Rain Weather Limestone Bedrock.
From www.slideserve.com
PPT Weathering, Erosion & Deposition PowerPoint Presentation ID4655832 Why Does Acid Rain Weather Limestone Bedrock What happens in a reaction between acid rain and limestone? Limestone is mostly made up of the mineral calcium carbonate (caco3). When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Limestone caves form due to the chemical weathering of limestone bedrock caused by natural acid present in. Why Does Acid Rain Weather Limestone Bedrock.
From mavink.com
Acid Rain Limestone Why Does Acid Rain Weather Limestone Bedrock What happens in a reaction between acid rain and limestone? As rain falls to the ground, it. Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the combined. When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. Limestone. Why Does Acid Rain Weather Limestone Bedrock.
From owlcation.com
Chemical Weathering A Great Natural Force Owlcation Why Does Acid Rain Weather Limestone Bedrock What happens in a reaction between acid rain and limestone? On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water due to acid rain than areas with igneous bedrock. Chemical weathering increases steadily upward in the weathered bedrock, with intervals of more intense weathering along fractures, documenting the. Why Does Acid Rain Weather Limestone Bedrock.
From www.dreamstime.com
Limestone Surface Eroded by Acid Rain. Stock Photo Image of naturally Why Does Acid Rain Weather Limestone Bedrock When sulfurous, sulfuric, and nitric acids in polluted air and rain react with the calcite in marble and limestone, the calcite dissolves. On the positive side, regions where the bedrock or soil contain large amounts of limestone are less likely to have polluted water due to acid rain than areas with igneous bedrock. Chemical weathering increases steadily upward in the. Why Does Acid Rain Weather Limestone Bedrock.