Copper Iodide And Sulfuric Acid . For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. Reducing ability of the halide ions increases as you go down the. Copper (i) iodide react with sulfuric acid to produce copper sulfate, iodine, hydrogen sulfide and water. In fact you get a brown. [ 9 ] in the laboratory however, copper(i) iodide is. The reaction is done with potassium manganate(vii) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. During the reaction, the manganate(vii). Iodide ions reduce the sulphuric acid to a mixture of products including hydrogen sulphide. Copper(i) iodide can be prepared by heating iodine and copper in concentrated hydroiodic acid. H 2 so 4 (l) + nai. The iodide ions are oxidised to iodine. Reaction of iodide ions with concentrated sulfuric acid. The reaction of sodium iodide and concentrated sulfuric acid is: For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced.
from www.nagwa.com
H 2 so 4 (l) + nai. In fact you get a brown. Reaction of iodide ions with concentrated sulfuric acid. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. The iodide ions are oxidised to iodine. The reaction is done with potassium manganate(vii) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. Iodide ions reduce the sulphuric acid to a mixture of products including hydrogen sulphide. Copper (i) iodide react with sulfuric acid to produce copper sulfate, iodine, hydrogen sulfide and water. The reaction of sodium iodide and concentrated sulfuric acid is: Copper(i) iodide can be prepared by heating iodine and copper in concentrated hydroiodic acid.
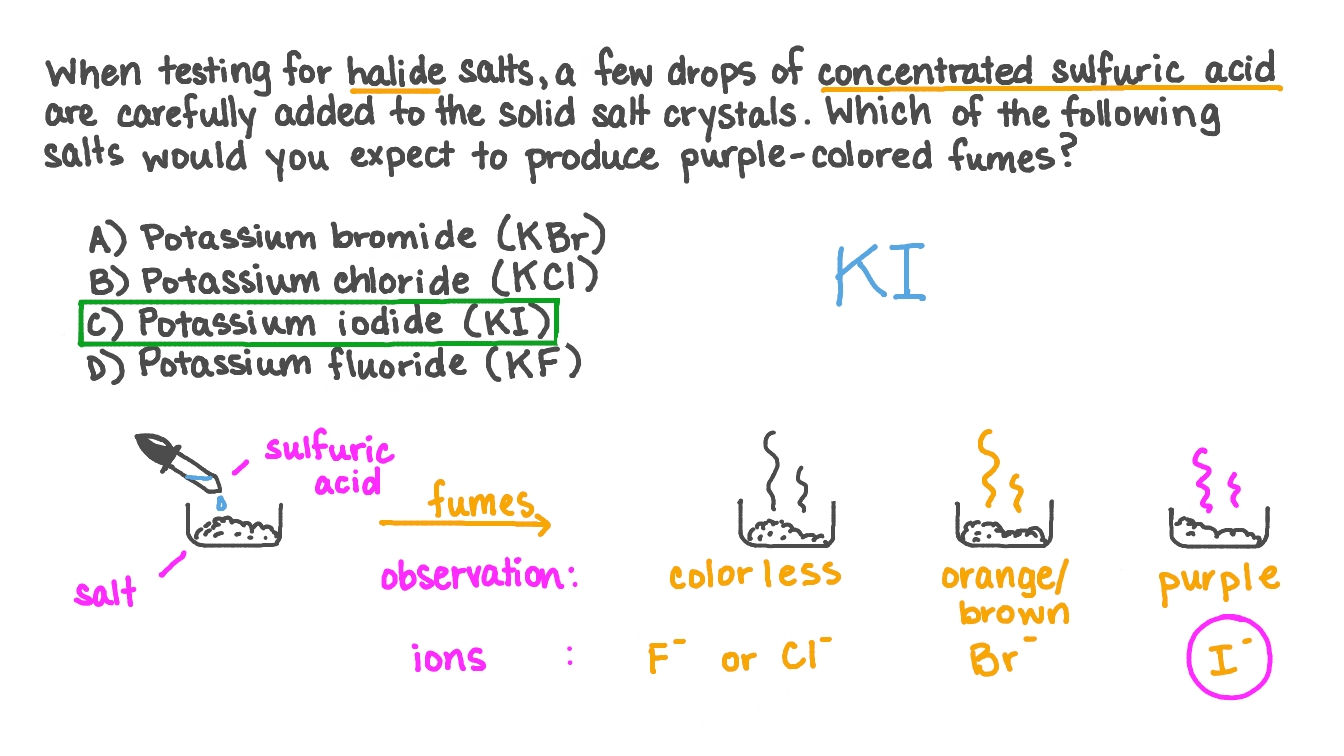
Question Video Identifying the Salt That Produces Purple Fumes upon
Copper Iodide And Sulfuric Acid Reaction of iodide ions with concentrated sulfuric acid. [ 9 ] in the laboratory however, copper(i) iodide is. Reaction of iodide ions with concentrated sulfuric acid. Reducing ability of the halide ions increases as you go down the. Iodide ions reduce the sulphuric acid to a mixture of products including hydrogen sulphide. During the reaction, the manganate(vii). Copper (i) iodide react with sulfuric acid to produce copper sulfate, iodine, hydrogen sulfide and water. H 2 so 4 (l) + nai. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Copper(i) iodide can be prepared by heating iodine and copper in concentrated hydroiodic acid. The reaction of sodium iodide and concentrated sulfuric acid is: The reaction is done with potassium manganate(vii) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. In fact you get a brown. The iodide ions are oxidised to iodine.
From www.numerade.com
SOLVED Aqueous copper(II) ion reacts with aqueous iodide ion to yield Copper Iodide And Sulfuric Acid For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. The reaction is done with potassium manganate(vii) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. In fact you get a brown. Reducing ability of the halide ions increases as you go down the.. Copper Iodide And Sulfuric Acid.
From www.youtube.com
Copper Sulfate and Potassium Iodide Solutions YouTube Copper Iodide And Sulfuric Acid The iodide ions are oxidised to iodine. Iodide ions reduce the sulphuric acid to a mixture of products including hydrogen sulphide. During the reaction, the manganate(vii). The reaction of sodium iodide and concentrated sulfuric acid is: In fact you get a brown. Reducing ability of the halide ions increases as you go down the. H 2 so 4 (l) +. Copper Iodide And Sulfuric Acid.
From www.youtube.com
Reaction of Copper sulfate (CuSO4) with Potassium iodide (KI) YouTube Copper Iodide And Sulfuric Acid [ 9 ] in the laboratory however, copper(i) iodide is. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. In fact you get a brown. The reaction of sodium iodide and concentrated sulfuric acid is: Copper(i) iodide can be prepared by heating iodine and. Copper Iodide And Sulfuric Acid.
From zelengarden.ru
Закончите схему реакции zn h2so4 конц s Copper Iodide And Sulfuric Acid Copper(i) iodide can be prepared by heating iodine and copper in concentrated hydroiodic acid. During the reaction, the manganate(vii). H 2 so 4 (l) + nai. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. [ 9 ] in the laboratory however, copper(i) iodide is.. Copper Iodide And Sulfuric Acid.
From www.researchgate.net
The reaction mechanism of sulfuric acid with polyethylene, introducing Copper Iodide And Sulfuric Acid Copper(i) iodide can be prepared by heating iodine and copper in concentrated hydroiodic acid. During the reaction, the manganate(vii). Copper (i) iodide react with sulfuric acid to produce copper sulfate, iodine, hydrogen sulfide and water. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. Reaction. Copper Iodide And Sulfuric Acid.
From www.alamy.com
Sulphuric acid reaction. Concentrated sulphuric acid being added to Copper Iodide And Sulfuric Acid The reaction of sodium iodide and concentrated sulfuric acid is: For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. Iodide ions reduce the sulphuric acid to a mixture of products including hydrogen sulphide. In fact you get a brown. H 2 so 4 (l) +. Copper Iodide And Sulfuric Acid.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记2.3.4 Halide Ion Reactions翰林国际教育 Copper Iodide And Sulfuric Acid Reaction of iodide ions with concentrated sulfuric acid. In fact you get a brown. Reducing ability of the halide ions increases as you go down the. Iodide ions reduce the sulphuric acid to a mixture of products including hydrogen sulphide. [ 9 ] in the laboratory however, copper(i) iodide is. During the reaction, the manganate(vii). H 2 so 4 (l). Copper Iodide And Sulfuric Acid.
From www.researchgate.net
Copper iodide catalyzed synthesis of aryl cyanide using benzyl cyanide Copper Iodide And Sulfuric Acid H 2 so 4 (l) + nai. Iodide ions reduce the sulphuric acid to a mixture of products including hydrogen sulphide. Copper (i) iodide react with sulfuric acid to produce copper sulfate, iodine, hydrogen sulfide and water. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and. Copper Iodide And Sulfuric Acid.
From brainly.in
Displacement reactions10. Aluminum + Sulfuric acid →11. Potassium Copper Iodide And Sulfuric Acid Reducing ability of the halide ions increases as you go down the. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. Iodide ions reduce the sulphuric acid to a mixture of products including hydrogen sulphide. During the reaction, the manganate(vii). The reaction is done with. Copper Iodide And Sulfuric Acid.
From www.slideserve.com
PPT Chapter 6 OxidationReduction Reactions PowerPoint Presentation Copper Iodide And Sulfuric Acid The iodide ions are oxidised to iodine. Reaction of iodide ions with concentrated sulfuric acid. During the reaction, the manganate(vii). Reducing ability of the halide ions increases as you go down the. [ 9 ] in the laboratory however, copper(i) iodide is. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a. Copper Iodide And Sulfuric Acid.
From www.nagwa.com
Question Video Identifying the Salt That Produces Purple Fumes upon Copper Iodide And Sulfuric Acid For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. Reducing ability of the halide ions increases as you go down the. In fact you get a brown. Reaction of iodide ions with concentrated sulfuric acid. Copper (i) iodide react with sulfuric acid to produce copper. Copper Iodide And Sulfuric Acid.
From www.compoundchem.com
Chemical Reactions Lead Iodide & ‘Golden Rain’ Compound Interest Copper Iodide And Sulfuric Acid The iodide ions are oxidised to iodine. Copper (i) iodide react with sulfuric acid to produce copper sulfate, iodine, hydrogen sulfide and water. Reaction of iodide ions with concentrated sulfuric acid. During the reaction, the manganate(vii). In fact you get a brown. Copper(i) iodide can be prepared by heating iodine and copper in concentrated hydroiodic acid. [ 9 ] in. Copper Iodide And Sulfuric Acid.
From www.chegg.com
Solved What Would Be The Proper Mechanism Of The Reaction... Copper Iodide And Sulfuric Acid During the reaction, the manganate(vii). The iodide ions are oxidised to iodine. Iodide ions reduce the sulphuric acid to a mixture of products including hydrogen sulphide. The reaction is done with potassium manganate(vii) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. [ 9 ] in the laboratory however, copper(i) iodide is. Reaction of iodide ions with concentrated sulfuric. Copper Iodide And Sulfuric Acid.
From www.researchgate.net
Hand specimen of the copper iodide and iodatebearing (redcolored Copper Iodide And Sulfuric Acid Reaction of iodide ions with concentrated sulfuric acid. [ 9 ] in the laboratory however, copper(i) iodide is. During the reaction, the manganate(vii). The reaction of sodium iodide and concentrated sulfuric acid is: The iodide ions are oxidised to iodine. Copper (i) iodide react with sulfuric acid to produce copper sulfate, iodine, hydrogen sulfide and water. In fact you get. Copper Iodide And Sulfuric Acid.
From www.indiamart.com
COPPER (I) IODIDE 98 Practical at Rs 993.5 Cuprous Iodide in Mumbai Copper Iodide And Sulfuric Acid Copper (i) iodide react with sulfuric acid to produce copper sulfate, iodine, hydrogen sulfide and water. Reaction of iodide ions with concentrated sulfuric acid. Reducing ability of the halide ions increases as you go down the. The reaction is done with potassium manganate(vii) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. For example, if you react copper(i) oxide. Copper Iodide And Sulfuric Acid.
From www.sciencephoto.com
Copper (I) iodide precipitate Stock Image A500/0573 Science Photo Copper Iodide And Sulfuric Acid For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. The reaction is done with potassium manganate(vii) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. Reducing ability of the halide ions increases as you go down the. Iodide ions reduce the sulphuric acid. Copper Iodide And Sulfuric Acid.
From www.youtube.com
CuSO4+KI=CuI+I2+K2SO4 Balanced EquationCopper sulphate+Potassium Copper Iodide And Sulfuric Acid Reaction of iodide ions with concentrated sulfuric acid. Reducing ability of the halide ions increases as you go down the. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. [ 9 ] in the laboratory however, copper(i) iodide is. Iodide ions reduce the sulphuric. Copper Iodide And Sulfuric Acid.
From www.researchgate.net
Single crystal Xray structures of Zn MOF having copper(I) iodide Copper Iodide And Sulfuric Acid Copper(i) iodide can be prepared by heating iodine and copper in concentrated hydroiodic acid. Iodide ions reduce the sulphuric acid to a mixture of products including hydrogen sulphide. Reaction of iodide ions with concentrated sulfuric acid. The reaction of sodium iodide and concentrated sulfuric acid is: Copper (i) iodide react with sulfuric acid to produce copper sulfate, iodine, hydrogen sulfide. Copper Iodide And Sulfuric Acid.
From www.semanticscholar.org
Figure 1 from Selective copper(II) acetate and potassium iodide Copper Iodide And Sulfuric Acid During the reaction, the manganate(vii). Copper(i) iodide can be prepared by heating iodine and copper in concentrated hydroiodic acid. [ 9 ] in the laboratory however, copper(i) iodide is. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Reducing ability of the halide ions. Copper Iodide And Sulfuric Acid.
From www.sciencephoto.com
Copper (I) iodide precipitate Stock Image C030/7215 Science Photo Copper Iodide And Sulfuric Acid For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. During the reaction, the manganate(vii). Copper(i) iodide can be prepared by heating iodine and copper in concentrated hydroiodic acid. In fact you get a brown. [ 9 ] in the laboratory however, copper(i) iodide is.. Copper Iodide And Sulfuric Acid.
From pubs.acs.org
A Copper Iodide ClusterBased Coordination Polymer as an Unconventional Copper Iodide And Sulfuric Acid Copper (i) iodide react with sulfuric acid to produce copper sulfate, iodine, hydrogen sulfide and water. During the reaction, the manganate(vii). [ 9 ] in the laboratory however, copper(i) iodide is. The reaction of sodium iodide and concentrated sulfuric acid is: The iodide ions are oxidised to iodine. In fact you get a brown. Copper(i) iodide can be prepared by. Copper Iodide And Sulfuric Acid.
From www.youtube.com
Neutralisation Reaction Sulfuric Acid and Sodium Hydroxide Balancing Copper Iodide And Sulfuric Acid [ 9 ] in the laboratory however, copper(i) iodide is. Copper (i) iodide react with sulfuric acid to produce copper sulfate, iodine, hydrogen sulfide and water. Reducing ability of the halide ions increases as you go down the. The reaction of sodium iodide and concentrated sulfuric acid is: Copper(i) iodide can be prepared by heating iodine and copper in concentrated. Copper Iodide And Sulfuric Acid.
From www.numerade.com
SOLVED 'Reaction of sodium dichromate with potassium iodide in Copper Iodide And Sulfuric Acid The iodide ions are oxidised to iodine. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Reaction of iodide ions. Copper Iodide And Sulfuric Acid.
From www.alamy.com
Brass analysis. Image 3 of 7. Potassium iodide was mixed with the Copper Iodide And Sulfuric Acid H 2 so 4 (l) + nai. Reaction of iodide ions with concentrated sulfuric acid. Iodide ions reduce the sulphuric acid to a mixture of products including hydrogen sulphide. Copper(i) iodide can be prepared by heating iodine and copper in concentrated hydroiodic acid. Reducing ability of the halide ions increases as you go down the. [ 9 ] in the. Copper Iodide And Sulfuric Acid.
From www.indiamart.com
Copper Iodide, Pharmaceutical Ingredients, Active Pharma Ingredients Copper Iodide And Sulfuric Acid During the reaction, the manganate(vii). For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. In fact you get a brown. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced.. Copper Iodide And Sulfuric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction sodium bromide iodide Copper Iodide And Sulfuric Acid H 2 so 4 (l) + nai. The reaction is done with potassium manganate(vii) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. During the reaction, the manganate(vii). [ 9 ] in the laboratory however, copper(i) iodide is. Reaction of iodide ions with concentrated sulfuric acid. Copper (i) iodide react with sulfuric acid to produce copper sulfate, iodine, hydrogen. Copper Iodide And Sulfuric Acid.
From ar.inspiredpencil.com
Nitric Acid And Sulfuric Acid Copper Iodide And Sulfuric Acid Copper(i) iodide can be prepared by heating iodine and copper in concentrated hydroiodic acid. The reaction of sodium iodide and concentrated sulfuric acid is: The iodide ions are oxidised to iodine. During the reaction, the manganate(vii). The reaction is done with potassium manganate(vii) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. [ 9 ] in the laboratory however,. Copper Iodide And Sulfuric Acid.
From www.semanticscholar.org
Figure 4 from Multiphoton absorption in lowdimensional cesium copper Copper Iodide And Sulfuric Acid During the reaction, the manganate(vii). H 2 so 4 (l) + nai. Copper(i) iodide can be prepared by heating iodine and copper in concentrated hydroiodic acid. The iodide ions are oxidised to iodine. Reaction of iodide ions with concentrated sulfuric acid. In fact you get a brown. Reducing ability of the halide ions increases as you go down the. Copper. Copper Iodide And Sulfuric Acid.
From www.researchgate.net
Iodoniums and copper catalysis oxidative addition to Cu(I) and Copper Iodide And Sulfuric Acid Reducing ability of the halide ions increases as you go down the. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. Reaction of iodide ions with concentrated sulfuric acid. Copper (i) iodide react with sulfuric acid to produce copper sulfate, iodine, hydrogen sulfide and water.. Copper Iodide And Sulfuric Acid.
From mavink.com
Sodium Thiosulfate Experiment Copper Iodide And Sulfuric Acid Reducing ability of the halide ions increases as you go down the. [ 9 ] in the laboratory however, copper(i) iodide is. Copper (i) iodide react with sulfuric acid to produce copper sulfate, iodine, hydrogen sulfide and water. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate. Copper Iodide And Sulfuric Acid.
From www.toppr.com
What happen when?Copper sulphate reacts with potassium iodide. Copper Iodide And Sulfuric Acid For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Copper(i) iodide can be prepared by heating iodine and copper in concentrated hydroiodic acid. Copper (i) iodide react with sulfuric acid to produce copper sulfate, iodine, hydrogen sulfide and water. The iodide ions are oxidised. Copper Iodide And Sulfuric Acid.
From www.youtube.com
Balancing Chemical Equations. Part 2 Sulfuric Acid and Sodium Copper Iodide And Sulfuric Acid Copper (i) iodide react with sulfuric acid to produce copper sulfate, iodine, hydrogen sulfide and water. H 2 so 4 (l) + nai. Copper(i) iodide can be prepared by heating iodine and copper in concentrated hydroiodic acid. The reaction is done with potassium manganate(vii) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. During the reaction, the manganate(vii). Reducing. Copper Iodide And Sulfuric Acid.
From www.youtube.com
Potassium Iodide and Sulphuric Acid YouTube Copper Iodide And Sulfuric Acid Copper(i) iodide can be prepared by heating iodine and copper in concentrated hydroiodic acid. H 2 so 4 (l) + nai. In fact you get a brown. For example, if you react copper(i) oxide with hot dilute sulphuric acid, you might expect to get a solution of copper(i) sulphate and water produced. Reaction of iodide ions with concentrated sulfuric acid.. Copper Iodide And Sulfuric Acid.
From meryes.weebly.com
Balanced chemical equation with state symbols calculator meryes Copper Iodide And Sulfuric Acid [ 9 ] in the laboratory however, copper(i) iodide is. Reducing ability of the halide ions increases as you go down the. During the reaction, the manganate(vii). Reaction of iodide ions with concentrated sulfuric acid. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. In. Copper Iodide And Sulfuric Acid.
From www.researchgate.net
Copper iodide/DABCO/PivOH catalyzed process for Copper Iodide And Sulfuric Acid The iodide ions are oxidised to iodine. The reaction is done with potassium manganate(vii) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. The reaction of sodium iodide and concentrated sulfuric acid is: For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and water. H 2. Copper Iodide And Sulfuric Acid.