Standard Specific Heat Of Water . Specific heat is the energy required to raise the temperature of a unit mass of a substance by. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific. 58 rows specific heat capacity of water. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Water’s specific heat is one of its most interesting characteristics. Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. If the mass of the substance is unity then the heat capacity is called specific heat capacity or the specific heat.
from studylib.net
58 rows specific heat capacity of water. Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific. If the mass of the substance is unity then the heat capacity is called specific heat capacity or the specific heat. Specific heat is the energy required to raise the temperature of a unit mass of a substance by. Water’s specific heat is one of its most interesting characteristics. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,.
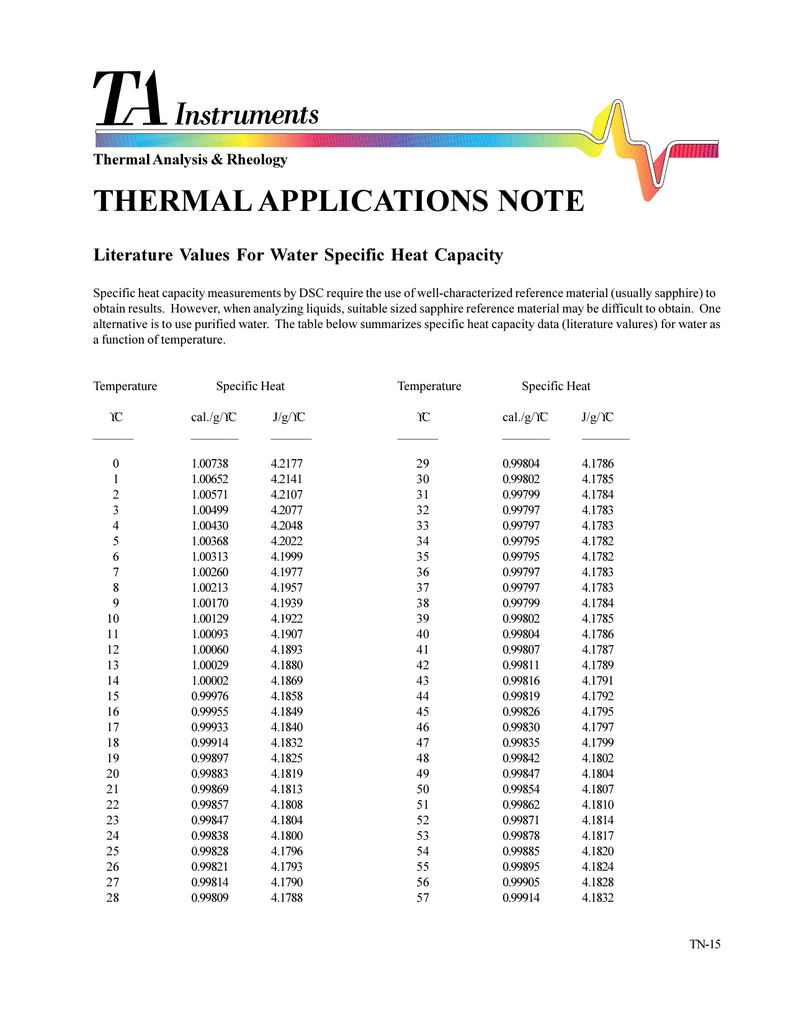
Literature values for water specific heat capacity, TN15
Standard Specific Heat Of Water Specific heat is the energy required to raise the temperature of a unit mass of a substance by. Water’s specific heat is one of its most interesting characteristics. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific. 58 rows specific heat capacity of water. Specific heat is the energy required to raise the temperature of a unit mass of a substance by. Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). If the mass of the substance is unity then the heat capacity is called specific heat capacity or the specific heat.
From missehonorsbio.blogspot.com
EC Honors Biology Specific Heat and Water as a Solvent Standard Specific Heat Of Water 58 rows specific heat capacity of water. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. If the mass of the substance is unity then the heat capacity is called specific heat capacity or the specific heat. Water’s specific heat is one of its most interesting characteristics. In this article, we’ll be. Standard Specific Heat Of Water.
From slideplayer.com
Specific Heat As a substance absorbs heat…it’s molecules begin moving Standard Specific Heat Of Water If the mass of the substance is unity then the heat capacity is called specific heat capacity or the specific heat. Water’s specific heat is one of its most interesting characteristics. Specific heat is the energy required to raise the temperature of a unit mass of a substance by. Specific heat is defined by the amount of heat needed to. Standard Specific Heat Of Water.
From ar.inspiredpencil.com
Specific Heat Of Water Chart Standard Specific Heat Of Water Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). If the mass of the substance is unity then the heat capacity is called specific heat capacity or the specific heat. Specific heat is the energy required to raise the temperature of a unit mass of. Standard Specific Heat Of Water.
From www.slideserve.com
PPT Find the change in entropy when 87.3 g of water vapor condense Standard Specific Heat Of Water Water’s specific heat is one of its most interesting characteristics. Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. 58 rows specific heat capacity of water. If the mass of. Standard Specific Heat Of Water.
From ar.inspiredpencil.com
Specific Heat Of Water Chart Standard Specific Heat Of Water If the mass of the substance is unity then the heat capacity is called specific heat capacity or the specific heat. In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,.. Standard Specific Heat Of Water.
From studylib.net
Literature values for water specific heat capacity, TN15 Standard Specific Heat Of Water If the mass of the substance is unity then the heat capacity is called specific heat capacity or the specific heat. Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. Water’s specific heat is one of its most interesting characteristics. In this article, we’ll be covering what. Standard Specific Heat Of Water.
From www.slideserve.com
PPT High Specific Heat of Water PowerPoint Presentation, free Standard Specific Heat Of Water Specific heat is the energy required to raise the temperature of a unit mass of a substance by. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Water’s specific heat is one of its most interesting characteristics. In this article, we’ll be covering what specific heat is, what equation you use to. Standard Specific Heat Of Water.
From ar.inspiredpencil.com
Heat Capacity Of Water Standard Specific Heat Of Water Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. Water’s specific heat is one of its most interesting characteristics. Specific heat is the. Standard Specific Heat Of Water.
From brainly.com
Consider the heating curve for water. A graph of the heating curve for Standard Specific Heat Of Water Specific heat is the energy required to raise the temperature of a unit mass of a substance by. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Water’s specific heat is one of its most interesting characteristics. Online calculator, figures and tables showing specific heat of liquid water at constant volume or. Standard Specific Heat Of Water.
From rechschem.weebly.com
Specific Heat Standard Specific Heat Of Water Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific. Specific heat is the energy required to raise the temperature of a unit mass of a substance by. Specific heat is. Standard Specific Heat Of Water.
From joiamykdj.blob.core.windows.net
What Heats Up Water at Alexander Dennison blog Standard Specific Heat Of Water Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. Specific heat is the energy required to raise the temperature of a unit mass of a substance by. Water’s specific heat is one of its most interesting characteristics. Specific heat is defined by the amount of heat needed. Standard Specific Heat Of Water.
From sractclx.blogspot.com
Specific Heat Capacity Water / schoolphysics The specific Standard Specific Heat Of Water If the mass of the substance is unity then the heat capacity is called specific heat capacity or the specific heat. Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. In this article, we’ll be covering what specific heat is, what equation you use to find specific. Standard Specific Heat Of Water.
From proper-cooking.info
Specific Heat Of Water Jgc Standard Specific Heat Of Water Specific heat is the energy required to raise the temperature of a unit mass of a substance by. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. Water’s specific heat. Standard Specific Heat Of Water.
From www.coursehero.com
[Solved] 1. Find the amount of heat needed to raise the temperature of Standard Specific Heat Of Water Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). 58 rows specific heat capacity of water. Water’s specific heat is one of its most interesting characteristics. If the mass of the substance is unity then the heat capacity is called specific heat capacity or the. Standard Specific Heat Of Water.
From www.tec-science.com
Specific heat capacity of selected substances tecscience Standard Specific Heat Of Water Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and. Standard Specific Heat Of Water.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Standard Specific Heat Of Water Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. Standard Specific Heat Of Water.
From www.chegg.com
Solved 1 A hot copper block is dropped into water in an Standard Specific Heat Of Water Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). If the mass of the substance is unity then the heat capacity is called specific heat capacity or the specific heat. Online calculator, figures and tables showing specific heat of liquid water at constant volume or. Standard Specific Heat Of Water.
From www.chegg.com
Solved When a solid dissolves in water, heat may be evolved Standard Specific Heat Of Water If the mass of the substance is unity then the heat capacity is called specific heat capacity or the specific heat. Specific heat is the energy required to raise the temperature of a unit mass of a substance by. In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s. Standard Specific Heat Of Water.
From studylib.net
Specific heat capacity Standard Specific Heat Of Water Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. 58 rows specific heat capacity of water. Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. Specific heat is the energy required to raise the temperature of a unit mass. Standard Specific Heat Of Water.
From www.chegg.com
Solved When a solid dissolves in water, heat may be evolved Standard Specific Heat Of Water In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific. Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. 58 rows specific heat capacity of water. Water’s specific heat is one of its most interesting. Standard Specific Heat Of Water.
From ar.inspiredpencil.com
Specific Heat Of Water Chart Standard Specific Heat Of Water Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). If the mass of the substance is unity then the heat capacity is called. Standard Specific Heat Of Water.
From www.semanticscholar.org
Table 9 from Specific heat of liquid ammonia Semantic Scholar Standard Specific Heat Of Water Water’s specific heat is one of its most interesting characteristics. In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. 58 rows specific heat capacity of water. Online calculator, figures and. Standard Specific Heat Of Water.
From www.animalia-life.club
Specific Heat Chart Of Common Substances Standard Specific Heat Of Water Specific heat is the energy required to raise the temperature of a unit mass of a substance by. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific. Online calculator, figures. Standard Specific Heat Of Water.
From www.numerade.com
SOLVED 100 g of water is heated from 30^∘C to 50^∘C ignoring the Standard Specific Heat Of Water Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. 58 rows specific heat capacity of water. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Water’s specific heat is one of its most interesting characteristics. In this. Standard Specific Heat Of Water.
From www.researchgate.net
Heat capacity of pure water as function of temperature. Line calculated Standard Specific Heat Of Water Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. Specific heat is the energy required to raise the temperature of a unit mass of a substance by. Water’s specific heat is one of its most interesting characteristics. 58 rows specific heat capacity of water. In this article,. Standard Specific Heat Of Water.
From studylib.net
1.3 Specific Heat Capacity Standard Specific Heat Of Water Specific heat is the energy required to raise the temperature of a unit mass of a substance by. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. 58 rows specific heat capacity of water. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of. Standard Specific Heat Of Water.
From mcv.essaytyper.cloudns.cx
Order Essay Services & Assignment Papers Online specific heat Standard Specific Heat Of Water Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). 58 rows specific heat capacity of water. If the mass of the substance is unity then the heat capacity is called specific heat capacity or the specific heat. Online calculator, figures and tables showing specific heat. Standard Specific Heat Of Water.
From www.saralmind.com
Heat Notes, Videos, QA and Tests Class 10>Science>Heat SaralMind Standard Specific Heat Of Water If the mass of the substance is unity then the heat capacity is called specific heat capacity or the specific heat. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree. Standard Specific Heat Of Water.
From ar.inspiredpencil.com
Specific Heat Of Water Chart Standard Specific Heat Of Water 58 rows specific heat capacity of water. Specific heat is the energy required to raise the temperature of a unit mass of a substance by. Water’s specific heat is one of its most interesting characteristics. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Thermal. Standard Specific Heat Of Water.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Standard Specific Heat Of Water Specific heat is the energy required to raise the temperature of a unit mass of a substance by. Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. Water’s specific heat is one of its most interesting characteristics. Thermal properties of water at different temperatures like density, freezing. Standard Specific Heat Of Water.
From whatsinsight.org
What is the Specific Heat of Water? What's Insight Standard Specific Heat Of Water Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Water’s specific heat is one of its most interesting characteristics. In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific. Specific heat is the energy required to raise the temperature of. Standard Specific Heat Of Water.
From www.slideserve.com
PPT Unit 09 PowerPoint Presentation, free download ID5621270 Standard Specific Heat Of Water Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. 58 rows specific heat capacity of water. Thermal properties of water at different temperatures like density, freezing temperature, boiling temperature, latent heat of melting,. Specific heat is defined by the amount of heat needed to raise the temperature. Standard Specific Heat Of Water.
From byjus.com
The SI unit of specific heat capacity of a substance is Standard Specific Heat Of Water Water’s specific heat is one of its most interesting characteristics. 58 rows specific heat capacity of water. Online calculator, figures and tables showing specific heat of liquid water at constant volume or constant pressure at temperatures from 0 to. In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s. Standard Specific Heat Of Water.
From www.coursehero.com
[Solved] 1. Find the amount of heat needed to raise the temperature of Standard Specific Heat Of Water Water’s specific heat is one of its most interesting characteristics. In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). Thermal properties of water. Standard Specific Heat Of Water.
From www.chegg.com
Solved When a solid dissolves in water, heat may be evolved Standard Specific Heat Of Water Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree celsius (°c). In this article, we’ll be covering what specific heat is, what equation you use to find specific heat, and why water’s specific. Online calculator, figures and tables showing specific heat of liquid water at constant volume. Standard Specific Heat Of Water.