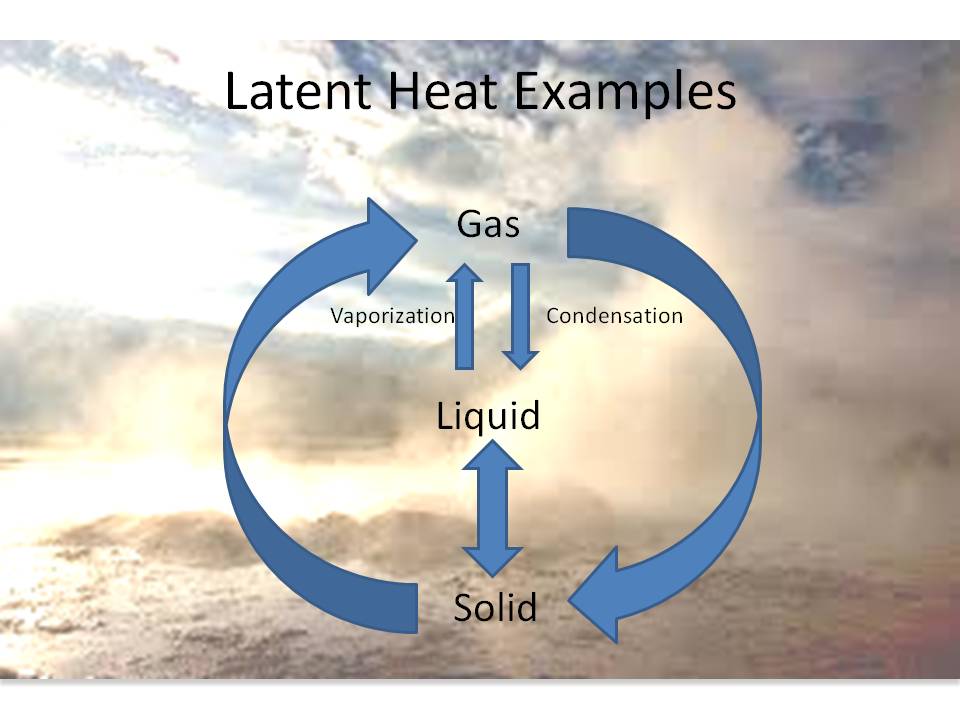
Why Latent Heat Is Called Hidden Heat . It could either be from a gas to a liquid or liquid to a solid and vice versa. During the transition of state, latent heat remains concealed. The latent heat is normally expressed as the amount of heat (in units of joules or calories) per mole or unit mass of the substance undergoing a change of state. Latent heat is related to a heat property called enthalpy. Heats of fusion and vaporization 1. Latent heat is defined as the heat or energy that is absorbed or released during a phase change of a substance. Because latent heat does not show temperature changes, it is referred to as hidden heat. Download complete chapter notes of thermodynamics. Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Phase changes can have a tremendous stabilizing effect even on. The heat required to change the phase of a substance without raising its temperature is called latent heat. Therefore, this energy is hidden within the. Literally, the term latent means hidden;
from www.bpiexamacademy.com
The latent heat is normally expressed as the amount of heat (in units of joules or calories) per mole or unit mass of the substance undergoing a change of state. Heats of fusion and vaporization 1. Download complete chapter notes of thermodynamics. Because latent heat does not show temperature changes, it is referred to as hidden heat. Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Literally, the term latent means hidden; It could either be from a gas to a liquid or liquid to a solid and vice versa. The heat required to change the phase of a substance without raising its temperature is called latent heat. Phase changes can have a tremendous stabilizing effect even on. Latent heat is defined as the heat or energy that is absorbed or released during a phase change of a substance.
What's the difference between latent and sensible heat on the BPI exam
Why Latent Heat Is Called Hidden Heat Phase changes can have a tremendous stabilizing effect even on. Because latent heat does not show temperature changes, it is referred to as hidden heat. Download complete chapter notes of thermodynamics. Latent heat is defined as the heat or energy that is absorbed or released during a phase change of a substance. Heats of fusion and vaporization 1. During the transition of state, latent heat remains concealed. Therefore, this energy is hidden within the. The heat required to change the phase of a substance without raising its temperature is called latent heat. The latent heat is normally expressed as the amount of heat (in units of joules or calories) per mole or unit mass of the substance undergoing a change of state. Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Literally, the term latent means hidden; Phase changes can have a tremendous stabilizing effect even on. It could either be from a gas to a liquid or liquid to a solid and vice versa. Latent heat is related to a heat property called enthalpy.
From www.youtube.com
What is Heat? Sensible Heat & Latent Heat Types, Formula, Units Why Latent Heat Is Called Hidden Heat During the transition of state, latent heat remains concealed. Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Because latent heat does not show temperature changes, it is referred to as hidden heat. Latent heat is defined as the heat or energy that is absorbed or. Why Latent Heat Is Called Hidden Heat.
From www.pinterest.com
INFO SHEET Hidden heat Converting a liquid at its boiling point to Why Latent Heat Is Called Hidden Heat Because latent heat does not show temperature changes, it is referred to as hidden heat. Latent heat is defined as the heat or energy that is absorbed or released during a phase change of a substance. Phase changes can have a tremendous stabilizing effect even on. The heat required to change the phase of a substance without raising its temperature. Why Latent Heat Is Called Hidden Heat.
From www.youtube.com
Sensible heat Difference between sensible and latent heat Latent Why Latent Heat Is Called Hidden Heat Literally, the term latent means hidden; It could either be from a gas to a liquid or liquid to a solid and vice versa. Latent heat is related to a heat property called enthalpy. The latent heat is normally expressed as the amount of heat (in units of joules or calories) per mole or unit mass of the substance undergoing. Why Latent Heat Is Called Hidden Heat.
From pediaa.com
Difference Between Latent Heat and Sensible Heat Definition Why Latent Heat Is Called Hidden Heat Latent heat is related to a heat property called enthalpy. The heat required to change the phase of a substance without raising its temperature is called latent heat. It could either be from a gas to a liquid or liquid to a solid and vice versa. During the transition of state, latent heat remains concealed. Heats of fusion and vaporization. Why Latent Heat Is Called Hidden Heat.
From www.slideserve.com
PPT Heat, Temperature, and Internal Energy PowerPoint Presentation Why Latent Heat Is Called Hidden Heat Download complete chapter notes of thermodynamics. Therefore, this energy is hidden within the. Latent heat is defined as the heat or energy that is absorbed or released during a phase change of a substance. Phase changes can have a tremendous stabilizing effect even on. Latent heat, energy absorbed or released by a substance during a change in its physical state. Why Latent Heat Is Called Hidden Heat.
From www.youtube.com
Latent heat YouTube Why Latent Heat Is Called Hidden Heat Download complete chapter notes of thermodynamics. Latent heat is related to a heat property called enthalpy. Therefore, this energy is hidden within the. Literally, the term latent means hidden; The latent heat is normally expressed as the amount of heat (in units of joules or calories) per mole or unit mass of the substance undergoing a change of state. It. Why Latent Heat Is Called Hidden Heat.
From askfilo.com
Latent heat (Hidden heat) It is observed that the temperature of the sy.. Why Latent Heat Is Called Hidden Heat Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. It could either be from a gas to a liquid or liquid to a solid and vice versa. Latent heat is related to a heat property called enthalpy. Phase changes can have a tremendous stabilizing effect even. Why Latent Heat Is Called Hidden Heat.
From eduinput.com
Latent heatDefinition, Types, Example, And Specific Latent Heat Why Latent Heat Is Called Hidden Heat During the transition of state, latent heat remains concealed. Literally, the term latent means hidden; Latent heat is related to a heat property called enthalpy. The latent heat is normally expressed as the amount of heat (in units of joules or calories) per mole or unit mass of the substance undergoing a change of state. Latent heat, energy absorbed or. Why Latent Heat Is Called Hidden Heat.
From studiousguy.com
9 Latent Heat Examples in Daily Life StudiousGuy Why Latent Heat Is Called Hidden Heat The heat required to change the phase of a substance without raising its temperature is called latent heat. Therefore, this energy is hidden within the. Latent heat is defined as the heat or energy that is absorbed or released during a phase change of a substance. Latent heat is related to a heat property called enthalpy. Heats of fusion and. Why Latent Heat Is Called Hidden Heat.
From www.studocu.com
101lec37 Blaaaa Lecture 37 Latent heat and specific heat What 9 s Why Latent Heat Is Called Hidden Heat Because latent heat does not show temperature changes, it is referred to as hidden heat. Literally, the term latent means hidden; It could either be from a gas to a liquid or liquid to a solid and vice versa. During the transition of state, latent heat remains concealed. Latent heat is defined as the heat or energy that is absorbed. Why Latent Heat Is Called Hidden Heat.
From apollo.nvu.vsc.edu
Latent Heats sublimation and deposition Why Latent Heat Is Called Hidden Heat Because latent heat does not show temperature changes, it is referred to as hidden heat. The heat required to change the phase of a substance without raising its temperature is called latent heat. Literally, the term latent means hidden; Phase changes can have a tremendous stabilizing effect even on. Therefore, this energy is hidden within the. Latent heat is defined. Why Latent Heat Is Called Hidden Heat.
From www.linstitute.net
IB DP Chemistry HL复习笔记1.2.5 Gas Law Relationships翰林国际教育 Why Latent Heat Is Called Hidden Heat It could either be from a gas to a liquid or liquid to a solid and vice versa. Download complete chapter notes of thermodynamics. Latent heat is defined as the heat or energy that is absorbed or released during a phase change of a substance. Phase changes can have a tremendous stabilizing effect even on. The latent heat is normally. Why Latent Heat Is Called Hidden Heat.
From www.sciencelearn.org.nz
Latent heat graph — Science Learning Hub Why Latent Heat Is Called Hidden Heat Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Phase changes can have a tremendous stabilizing effect even on. During the transition of state, latent heat remains concealed. Therefore, this energy is hidden within the. Literally, the term latent means hidden; The latent heat is normally. Why Latent Heat Is Called Hidden Heat.
From scychiller.com
Understanding What Is BTU In Heat Measurement » Industrial Water Why Latent Heat Is Called Hidden Heat During the transition of state, latent heat remains concealed. The heat required to change the phase of a substance without raising its temperature is called latent heat. Because latent heat does not show temperature changes, it is referred to as hidden heat. Literally, the term latent means hidden; Latent heat is defined as the heat or energy that is absorbed. Why Latent Heat Is Called Hidden Heat.
From www.researchgate.net
Latent heat and melting temperature of PCM composites A reduction of Why Latent Heat Is Called Hidden Heat Literally, the term latent means hidden; Heats of fusion and vaporization 1. During the transition of state, latent heat remains concealed. Download complete chapter notes of thermodynamics. Latent heat is related to a heat property called enthalpy. The heat required to change the phase of a substance without raising its temperature is called latent heat. Phase changes can have a. Why Latent Heat Is Called Hidden Heat.
From www.tec-science.com
Why steam burns are more dangerous than water burns? tecscience Why Latent Heat Is Called Hidden Heat During the transition of state, latent heat remains concealed. Latent heat is defined as the heat or energy that is absorbed or released during a phase change of a substance. Literally, the term latent means hidden; Therefore, this energy is hidden within the. Heats of fusion and vaporization 1. Latent heat is related to a heat property called enthalpy. Latent. Why Latent Heat Is Called Hidden Heat.
From savree.com
Heat Explained saVRee Why Latent Heat Is Called Hidden Heat It could either be from a gas to a liquid or liquid to a solid and vice versa. Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Phase changes can have a tremendous stabilizing effect even on. Latent heat is defined as the heat or energy. Why Latent Heat Is Called Hidden Heat.
From www.youtube.com
Sensible heat vs latent heat / Tamil / Engineering concepts YouTube Why Latent Heat Is Called Hidden Heat Literally, the term latent means hidden; Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. It could either be from a gas to a liquid or liquid to a solid and vice versa. Phase changes can have a tremendous stabilizing effect even on. The heat required. Why Latent Heat Is Called Hidden Heat.
From mmerevise.co.uk
Specific Latent Heat Questions and Revision MME Why Latent Heat Is Called Hidden Heat Because latent heat does not show temperature changes, it is referred to as hidden heat. Phase changes can have a tremendous stabilizing effect even on. The latent heat is normally expressed as the amount of heat (in units of joules or calories) per mole or unit mass of the substance undergoing a change of state. Heats of fusion and vaporization. Why Latent Heat Is Called Hidden Heat.
From www.studypool.com
SOLUTION Definition of latent heat types and examples of problems Why Latent Heat Is Called Hidden Heat Heats of fusion and vaporization 1. Therefore, this energy is hidden within the. Because latent heat does not show temperature changes, it is referred to as hidden heat. The latent heat is normally expressed as the amount of heat (in units of joules or calories) per mole or unit mass of the substance undergoing a change of state. The heat. Why Latent Heat Is Called Hidden Heat.
From klafvmutm.blob.core.windows.net
Heat Source For Evaporation at Salvatore Shorey blog Why Latent Heat Is Called Hidden Heat Latent heat is defined as the heat or energy that is absorbed or released during a phase change of a substance. Because latent heat does not show temperature changes, it is referred to as hidden heat. Therefore, this energy is hidden within the. Heats of fusion and vaporization 1. Phase changes can have a tremendous stabilizing effect even on. Latent. Why Latent Heat Is Called Hidden Heat.
From www.nagwa.com
Lesson Specific Latent Heat Nagwa Why Latent Heat Is Called Hidden Heat Heats of fusion and vaporization 1. Latent heat is related to a heat property called enthalpy. Download complete chapter notes of thermodynamics. The heat required to change the phase of a substance without raising its temperature is called latent heat. Literally, the term latent means hidden; The latent heat is normally expressed as the amount of heat (in units of. Why Latent Heat Is Called Hidden Heat.
From thecontentauthority.com
Latent Heat Words 101+ Words Related To Latent Heat Why Latent Heat Is Called Hidden Heat Literally, the term latent means hidden; Phase changes can have a tremendous stabilizing effect even on. The heat required to change the phase of a substance without raising its temperature is called latent heat. Therefore, this energy is hidden within the. Because latent heat does not show temperature changes, it is referred to as hidden heat. Latent heat is related. Why Latent Heat Is Called Hidden Heat.
From gamesmartz.com
Latent Heat Definition & Image GameSmartz Why Latent Heat Is Called Hidden Heat Heats of fusion and vaporization 1. Latent heat is defined as the heat or energy that is absorbed or released during a phase change of a substance. Download complete chapter notes of thermodynamics. It could either be from a gas to a liquid or liquid to a solid and vice versa. The heat required to change the phase of a. Why Latent Heat Is Called Hidden Heat.
From www.slideserve.com
PPT Heat and Thermochemistry Review PowerPoint Presentation, free Why Latent Heat Is Called Hidden Heat During the transition of state, latent heat remains concealed. Heats of fusion and vaporization 1. Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Latent heat is related to a heat property called enthalpy. The heat required to change the phase of a substance without raising. Why Latent Heat Is Called Hidden Heat.
From www.slideserve.com
PPT 4.3 SPECIFIC LATENT HEAT PowerPoint Presentation, free download Why Latent Heat Is Called Hidden Heat It could either be from a gas to a liquid or liquid to a solid and vice versa. Latent heat is defined as the heat or energy that is absorbed or released during a phase change of a substance. Therefore, this energy is hidden within the. Latent heat is related to a heat property called enthalpy. During the transition of. Why Latent Heat Is Called Hidden Heat.
From www.slideserve.com
PPT Heat and Temperature PowerPoint Presentation, free download ID Why Latent Heat Is Called Hidden Heat Because latent heat does not show temperature changes, it is referred to as hidden heat. During the transition of state, latent heat remains concealed. It could either be from a gas to a liquid or liquid to a solid and vice versa. The latent heat is normally expressed as the amount of heat (in units of joules or calories) per. Why Latent Heat Is Called Hidden Heat.
From www.youtube.com
"Understanding Sensible Heat vs. Latent Heat What's the Difference Why Latent Heat Is Called Hidden Heat It could either be from a gas to a liquid or liquid to a solid and vice versa. Because latent heat does not show temperature changes, it is referred to as hidden heat. Heats of fusion and vaporization 1. Phase changes can have a tremendous stabilizing effect even on. Latent heat is defined as the heat or energy that is. Why Latent Heat Is Called Hidden Heat.
From www.slideserve.com
PPT RDI Product Training PowerPoint Presentation, free download ID Why Latent Heat Is Called Hidden Heat The latent heat is normally expressed as the amount of heat (in units of joules or calories) per mole or unit mass of the substance undergoing a change of state. Download complete chapter notes of thermodynamics. During the transition of state, latent heat remains concealed. Phase changes can have a tremendous stabilizing effect even on. Literally, the term latent means. Why Latent Heat Is Called Hidden Heat.
From www.diffzy.com
Specific Heat vs. Latent Heat What's The Difference (With Table) Why Latent Heat Is Called Hidden Heat Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Literally, the term latent means hidden; Heats of fusion and vaporization 1. Download complete chapter notes of thermodynamics. It could either be from a gas to a liquid or liquid to a solid and vice versa. Phase. Why Latent Heat Is Called Hidden Heat.
From www.youtube.com
8 Water Vapor and Latent Heat YouTube Why Latent Heat Is Called Hidden Heat It could either be from a gas to a liquid or liquid to a solid and vice versa. Latent heat is defined as the heat or energy that is absorbed or released during a phase change of a substance. Download complete chapter notes of thermodynamics. Latent heat is related to a heat property called enthalpy. Phase changes can have a. Why Latent Heat Is Called Hidden Heat.
From www.linstitute.net
AQA A Level Physics复习笔记6.4.4 Latent Heat Capacity翰林国际教育 Why Latent Heat Is Called Hidden Heat Therefore, this energy is hidden within the. Phase changes can have a tremendous stabilizing effect even on. Download complete chapter notes of thermodynamics. Literally, the term latent means hidden; Latent heat is defined as the heat or energy that is absorbed or released during a phase change of a substance. It could either be from a gas to a liquid. Why Latent Heat Is Called Hidden Heat.
From www.bpiexamacademy.com
What's the difference between latent and sensible heat on the BPI exam Why Latent Heat Is Called Hidden Heat Latent heat, energy absorbed or released by a substance during a change in its physical state (phase) that occurs without changing its temperature. Latent heat is defined as the heat or energy that is absorbed or released during a phase change of a substance. The latent heat is normally expressed as the amount of heat (in units of joules or. Why Latent Heat Is Called Hidden Heat.
From www.slideserve.com
PPT 4.3 SPECIFIC LATENT HEAT PowerPoint Presentation, free download Why Latent Heat Is Called Hidden Heat The latent heat is normally expressed as the amount of heat (in units of joules or calories) per mole or unit mass of the substance undergoing a change of state. Heats of fusion and vaporization 1. The heat required to change the phase of a substance without raising its temperature is called latent heat. Because latent heat does not show. Why Latent Heat Is Called Hidden Heat.
From www.ces.fau.edu
Climate Science Investigations South Florida Energy The Driver of Why Latent Heat Is Called Hidden Heat Latent heat is related to a heat property called enthalpy. The latent heat is normally expressed as the amount of heat (in units of joules or calories) per mole or unit mass of the substance undergoing a change of state. Literally, the term latent means hidden; Because latent heat does not show temperature changes, it is referred to as hidden. Why Latent Heat Is Called Hidden Heat.