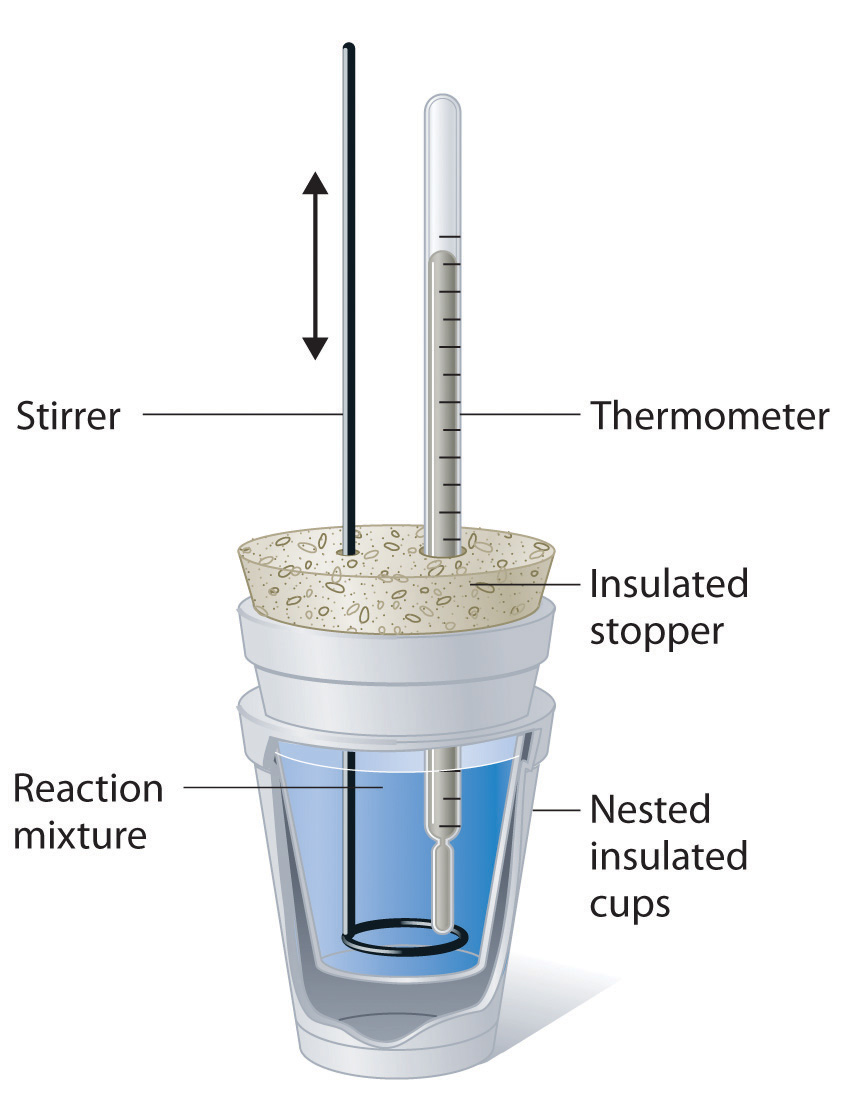
Chemistry Pressure Calorimeter . learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. Find examples, equations, and exercises for constant. learn how to measure the enthalpy change of a reaction using a calorimeter under constant pressure. This text is adapted from openstax, chemistry 2e, section 5.2: the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. See examples, formulas, and practice problems with. constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant.
from users.highland.edu
learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. learn how to measure the enthalpy change of a reaction using a calorimeter under constant pressure. See examples, formulas, and practice problems with. Find examples, equations, and exercises for constant. This text is adapted from openstax, chemistry 2e, section 5.2:
Calorimetry
Chemistry Pressure Calorimeter Find examples, equations, and exercises for constant. This text is adapted from openstax, chemistry 2e, section 5.2: learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. learn how to measure the enthalpy change of a reaction using a calorimeter under constant pressure. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. See examples, formulas, and practice problems with. learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Find examples, equations, and exercises for constant. constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant.
From exovxxbwq.blob.core.windows.net
Calorimeter In Chemical Engineering at Elizabeth Hodgson blog Chemistry Pressure Calorimeter constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant. See examples, formulas, and practice problems with. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. learn how to measure the enthalpy change of a. Chemistry Pressure Calorimeter.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Chemistry Pressure Calorimeter See examples, formulas, and practice problems with. learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change. Chemistry Pressure Calorimeter.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Chemistry Pressure Calorimeter constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant. learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. learn how. Chemistry Pressure Calorimeter.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Chemistry Pressure Calorimeter learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. This text is adapted from openstax, chemistry 2e, section 5.2: Find examples, equations, and exercises for constant. learn how to measure the enthalpy change of a reaction using a calorimeter under constant pressure. See examples, formulas, and practice problems with. constant pressure calorimetry is. Chemistry Pressure Calorimeter.
From www.fountainheadpress.com
Chemistry Illustrations Chemistry Pressure Calorimeter learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. Find examples, equations, and exercises for constant. This text is adapted from openstax, chemistry 2e, section 5.2: learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. See examples, formulas, and practice problems with. constant pressure. Chemistry Pressure Calorimeter.
From www.researchgate.net
Schematic of constant pressure calorimeter for APW classification Chemistry Pressure Calorimeter See examples, formulas, and practice problems with. This text is adapted from openstax, chemistry 2e, section 5.2: Find examples, equations, and exercises for constant. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure. Chemistry Pressure Calorimeter.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3207088 Chemistry Pressure Calorimeter This text is adapted from openstax, chemistry 2e, section 5.2: learn how to measure the enthalpy change of a reaction using a calorimeter under constant pressure. Find examples, equations, and exercises for constant. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. See examples, formulas, and practice problems with.. Chemistry Pressure Calorimeter.
From labsuppliesusa.com
Calorimeter Electric KLM Bio Scientific Chemistry Pressure Calorimeter constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant. Find examples, equations, and exercises for constant. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. the calorimeters described are designed to operate at constant. Chemistry Pressure Calorimeter.
From exosbbnfj.blob.core.windows.net
Calorimetry Ap Chemistry at Michael Faust blog Chemistry Pressure Calorimeter See examples, formulas, and practice problems with. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. This text is adapted from openstax, chemistry 2e, section 5.2: constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant.. Chemistry Pressure Calorimeter.
From www.chemistryspace.com
ThermoChemistry Calorimetry Chemistry Pressure Calorimeter the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. See examples, formulas, and practice problems with. . Chemistry Pressure Calorimeter.
From chem.libretexts.org
3.4 Calorimetry Chemistry LibreTexts Chemistry Pressure Calorimeter This text is adapted from openstax, chemistry 2e, section 5.2: the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. learn how to measure the enthalpy change of a reaction using. Chemistry Pressure Calorimeter.
From app.jove.com
Constant Pressure Calorimeter/ Coffee Cup Calorimeter Concept Chemistry Pressure Calorimeter learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. learn how to measure the enthalpy change of a reaction using a calorimeter under constant pressure. See examples, formulas, and practice problems with. Find examples, equations, and exercises for constant. This text is adapted from openstax, chemistry 2e, section 5.2: the calorimeters described are. Chemistry Pressure Calorimeter.
From studythalassian.z4.web.core.windows.net
How To Calculate Calorimeter Chemistry Pressure Calorimeter learn how to measure the enthalpy change of a reaction using a calorimeter under constant pressure. constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow.. Chemistry Pressure Calorimeter.
From www.w3schools.blog
Cp, Cv calorimetry W3schools Chemistry Pressure Calorimeter This text is adapted from openstax, chemistry 2e, section 5.2: learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. See examples, formulas, and practice problems with. Find examples, equations, and exercises for constant. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. constant pressure. Chemistry Pressure Calorimeter.
From ecampusontario.pressbooks.pub
5.2 Calorimetry Chemistry Chemistry Pressure Calorimeter This text is adapted from openstax, chemistry 2e, section 5.2: learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant. See examples, formulas, and practice problems with.. Chemistry Pressure Calorimeter.
From www.vrogue.co
Chemistry 101 Constant Pressure Calorimetry Youtube vrogue.co Chemistry Pressure Calorimeter This text is adapted from openstax, chemistry 2e, section 5.2: learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. See examples, formulas, and practice problems with. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. constant pressure calorimetry is a technique used to measure. Chemistry Pressure Calorimeter.
From www.shaalaa.com
Explain the construction of a calorimeter. Draw the necessary figure Chemistry Pressure Calorimeter learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. See examples, formulas, and practice problems with. learn how to measure the enthalpy change of a reaction using a calorimeter under constant pressure. learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. This text is. Chemistry Pressure Calorimeter.
From scienceinfo.com
Calorimeter Definition, Types and Uses Chemistry Pressure Calorimeter the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. See examples, formulas, and practice problems with. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. This text is adapted from openstax, chemistry 2e, section 5.2: learn how to measure. Chemistry Pressure Calorimeter.
From www.pearson.com
Chapter 09 16 Constant Pressure Calorimetry (coffee cup) Channels Chemistry Pressure Calorimeter constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. learn how to measure the enthalpy change of a reaction using a calorimeter under constant pressure.. Chemistry Pressure Calorimeter.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Chemistry Pressure Calorimeter See examples, formulas, and practice problems with. This text is adapted from openstax, chemistry 2e, section 5.2: the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Find examples, equations, and exercises for constant. constant pressure. Chemistry Pressure Calorimeter.
From www.reddit.com
How exactly is this calorimeter an example of constantpressure Chemistry Pressure Calorimeter learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. learn how. Chemistry Pressure Calorimeter.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Chemistry Pressure Calorimeter constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant. learn how to measure the enthalpy change of a reaction using a calorimeter under constant pressure. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions.. Chemistry Pressure Calorimeter.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Chemistry Pressure Calorimeter learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. This text is adapted from openstax, chemistry 2e, section 5.2: constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant. the calorimeters described are designed to operate at constant (atmospheric) pressure. Chemistry Pressure Calorimeter.
From users.highland.edu
Calorimetry Chemistry Pressure Calorimeter This text is adapted from openstax, chemistry 2e, section 5.2: constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. the calorimeters described are designed to. Chemistry Pressure Calorimeter.
From www.thoughtco.com
Calorimeter Definition in Chemistry Chemistry Pressure Calorimeter This text is adapted from openstax, chemistry 2e, section 5.2: learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. learn how to measure and calculate heat and enthalpy changes using. Chemistry Pressure Calorimeter.
From www.slideserve.com
PPT A calorimeter is used to measure the amount of heat absorbed or Chemistry Pressure Calorimeter constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant. learn how to measure the enthalpy change of a reaction using a calorimeter under constant pressure. learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. the calorimeters described are. Chemistry Pressure Calorimeter.
From thermonine92.blogspot.com
Thermochemistry Calorimeter Chemistry Pressure Calorimeter the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. Find examples, equations, and exercises for constant. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. constant pressure calorimetry is a technique used to measure the heat of a chemical. Chemistry Pressure Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Chemistry Pressure Calorimeter learn how to measure the enthalpy change of a reaction using a calorimeter under constant pressure. Find examples, equations, and exercises for constant. learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. This text is adapted from openstax, chemistry 2e, section 5.2: constant pressure calorimetry is a technique used to measure the heat. Chemistry Pressure Calorimeter.
From rumble.com
Calorimetry, Constant Pressure, Thermodynamics Chemistry Chemistry Pressure Calorimeter constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant. learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. learn how. Chemistry Pressure Calorimeter.
From www.youtube.com
AP Chemistry Thermochemistry I Part 4 Constant Pressure Calorimetry Chemistry Pressure Calorimeter learn how to measure the enthalpy change of a reaction using a calorimeter under constant pressure. Find examples, equations, and exercises for constant. constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant. the calorimeters described are designed to operate at constant (atmospheric) pressure. Chemistry Pressure Calorimeter.
From chemistrytalk.org
Calorimetry ChemTalk Chemistry Pressure Calorimeter constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. the calorimeters. Chemistry Pressure Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Chemistry Pressure Calorimeter This text is adapted from openstax, chemistry 2e, section 5.2: the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. Find examples, equations, and exercises for constant. constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant.. Chemistry Pressure Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Chemistry Pressure Calorimeter See examples, formulas, and practice problems with. learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. learn how to measure the enthalpy change of a reaction using a calorimeter under constant pressure. Find examples, equations, and exercises for constant. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical. Chemistry Pressure Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Chemistry Pressure Calorimeter constant pressure calorimetry is a technique used to measure the heat of a chemical reaction or a physical change under conditions of constant. learn how to measure the enthalpy change of a reaction using a calorimeter under constant pressure. See examples, formulas, and practice problems with. This text is adapted from openstax, chemistry 2e, section 5.2: learn. Chemistry Pressure Calorimeter.
From www.youtube.com
Constantpressure calorimetry Thermodynamics AP Chemistry Khan Chemistry Pressure Calorimeter See examples, formulas, and practice problems with. learn how to use calorimetry to measure heat transfer and enthalpy changes in chemical reactions and phase transitions. the calorimeters described are designed to operate at constant (atmospheric) pressure and are convenient to measure heat flow. Find examples, equations, and exercises for constant. learn how to measure the enthalpy change. Chemistry Pressure Calorimeter.