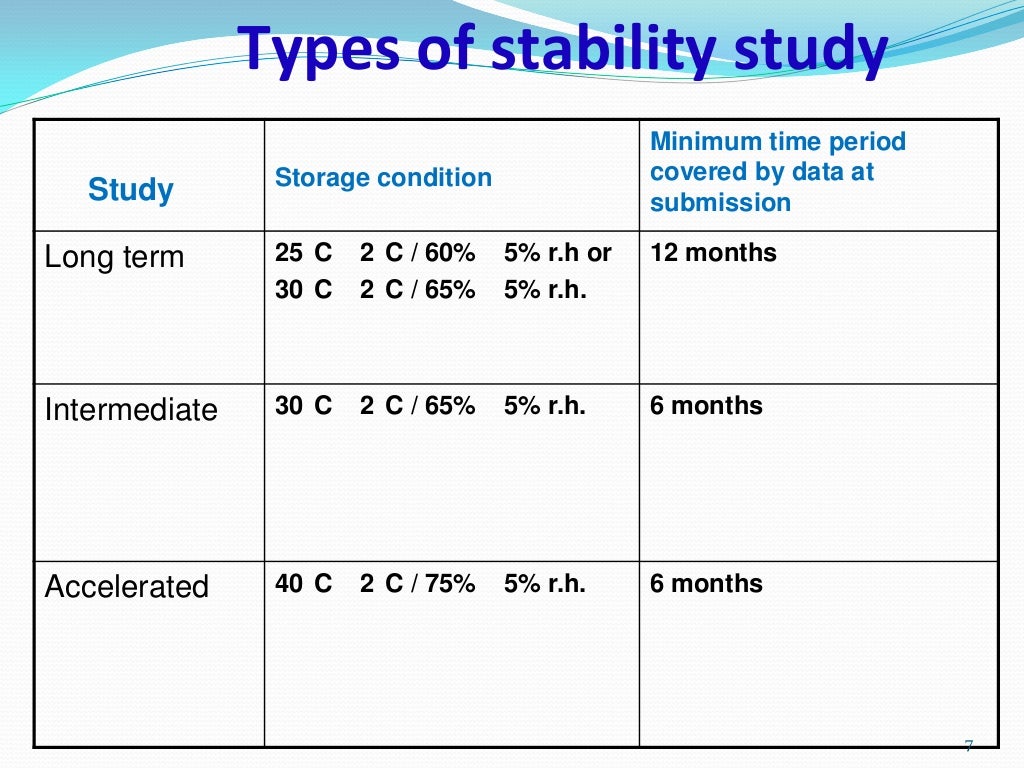
Stability Testing Time Points . Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. This guideline is intended to provide recommendations on how to use stability data generated in accordance with the principles. Sterile drug product batches (supporting anda submission) should be manufactured in a sterile facility. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the.
from www.slideshare.net
This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. This guideline is intended to provide recommendations on how to use stability data generated in accordance with the principles. Sterile drug product batches (supporting anda submission) should be manufactured in a sterile facility. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety.
Seminor on accelerated stability testing of dosage forms sahil
Stability Testing Time Points The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. Sterile drug product batches (supporting anda submission) should be manufactured in a sterile facility. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. This guideline is intended to provide recommendations on how to use stability data generated in accordance with the principles.
From www.researchgate.net
Normalised results of the shortterm stability testing at 40°C of BCR Stability Testing Time Points Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. Sterile drug product batches (supporting anda submission) should be manufactured in a sterile. Stability Testing Time Points.
From ipacanada.com
Stability Studies and Testing of Pharmaceuticals An Overview IPA Stability Testing Time Points The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. The purpose of stability testing is to provide evidence on how the. Stability Testing Time Points.
From q1scientific.com
A Q&A guide to stability storage Q1 Scientific Stability Testing Time Points The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. The purpose of stability testing is to provide. Stability Testing Time Points.
From www.researchgate.net
Stability test of the equilibrium point (1,1,1). Download Scientific Stability Testing Time Points This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. Sterile drug product batches (supporting anda submission) should be manufactured in a sterile facility. This guideline is intended to provide recommendations on how to use stability data generated in accordance with the principles. The purpose of stability. Stability Testing Time Points.
From www.slideserve.com
PPT DRUG STABILITY PowerPoint Presentation, free download ID12200727 Stability Testing Time Points Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. This guideline is intended to provide recommendations on how to use. Stability Testing Time Points.
From www.geeksforgeeks.org
Stability Testing Software Testing Stability Testing Time Points Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. The purpose of stability testing is to provide evidence on how the quality. Stability Testing Time Points.
From www.slideserve.com
PPT Order Of Reaction PowerPoint Presentation, free download ID Stability Testing Time Points The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. Sterile drug product batches (supporting anda submission) should. Stability Testing Time Points.
From www.slideserve.com
PPT STABILITY STUDIES PowerPoint Presentation ID217866 Stability Testing Time Points Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. Sterile drug product batches (supporting anda submission) should be manufactured in. Stability Testing Time Points.
From www.slideshare.net
Seminor on accelerated stability testing of dosage forms sahil Stability Testing Time Points The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. This guideline is intended to provide recommendations on how to use stability data generated in accordance with the principles. The purpose of stability testing is to provide evidence on how the quality of. Stability Testing Time Points.
From www.slideserve.com
PPT Stability Testing PowerPoint Presentation, free download ID481423 Stability Testing Time Points The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. Sterile drug product batches (supporting anda submission) should be manufactured in a sterile facility. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug. Stability Testing Time Points.
From www.researchgate.net
Stability studies. At each time point (0, 5, 10, 15, and 30 days), the Stability Testing Time Points The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. The purpose of stability testing is to provide evidence of. Stability Testing Time Points.
From ssics.in
Stability Testing Sure System Industrial Consultancy Service Stability Testing Time Points Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. This guideline is intended to provide recommendations on how to use stability data generated in accordance with the principles. Sterile drug product batches (supporting anda submission) should be manufactured in a sterile facility. The purpose of. Stability Testing Time Points.
From www.researchgate.net
Numerical stability test. Download Scientific Diagram Stability Testing Time Points The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. This document defines the stability data package for a new drug. Stability Testing Time Points.
From www.slideshare.net
ICH Guidelines for stability testing Stability Testing Time Points This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. This guideline is intended to provide recommendations on how to. Stability Testing Time Points.
From www.abtosoftware.com
OCR technology to automate stability testing Stability Testing Time Points Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. The purpose of stability testing is to provide evidence. Stability Testing Time Points.
From www.complifegroup.com
Stability test Complife Group Stability Testing Time Points The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. This guideline is intended to provide recommendations on how to use stability. Stability Testing Time Points.
From clinicalgate.com
Product stability and stability testing Clinical Gate Stability Testing Time Points Sterile drug product batches (supporting anda submission) should be manufactured in a sterile facility. Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under. Stability Testing Time Points.
From www.slideserve.com
PPT Stability Testing PowerPoint Presentation, free download ID481423 Stability Testing Time Points This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. Sterile drug product batches (supporting anda submission) should be manufactured in a sterile. Stability Testing Time Points.
From www.researchgate.net
Physical test comparison of Real Time stability analysis Download Table Stability Testing Time Points The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. This guideline is intended to provide recommendations on how to use stability data generated in accordance with the principles. Sterile drug product batches (supporting anda submission) should be manufactured in a sterile facility. The purpose. Stability Testing Time Points.
From www.researchgate.net
The Sensitivity of the GStability Testing Method. Download Stability Testing Time Points The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. The purpose of stability testing is to provide evidence of how the. Stability Testing Time Points.
From www.slideshare.net
Ich guidelines for stability studies 1 Stability Testing Time Points This guideline is intended to provide recommendations on how to use stability data generated in accordance with the principles. Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. The purpose of stability testing is to provide evidence on how the quality of a drug substance. Stability Testing Time Points.
From www.lyophilization.com
ICH Guidelines for Stability Testing Services LSNE Stability Testing Time Points Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. Sterile drug product batches (supporting anda submission) should be manufactured in a sterile facility. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under. Stability Testing Time Points.
From u-tor.com
What is Stability Testing? A Practical Guide with Example UTOR Stability Testing Time Points Sterile drug product batches (supporting anda submission) should be manufactured in a sterile facility. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. This guideline is intended to provide recommendations on how to use stability data generated in accordance with the principles.. Stability Testing Time Points.
From www.slideshare.net
Ich guideline for stability testing Stability Testing Time Points The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. Stability schedule such that only samples on the extremes of. Stability Testing Time Points.
From www.pharmaspecialists.com
Guidelines for Stability Testing of Pharmaceutical Products Stability Testing Time Points The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. The purpose of stability testing is to provide evidence of. Stability Testing Time Points.
From www.researchgate.net
Longterm stability testing for three different ETMs. Download Stability Testing Time Points Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. Sterile drug product batches (supporting anda submission) should be manufactured in a sterile facility. This guideline is intended to provide recommendations on how to use stability data generated in accordance with the principles. The purpose of. Stability Testing Time Points.
From deacom.com
Stability Testing with ERP Software ERP Stability Testing Time Points This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. Sterile drug product batches (supporting anda submission) should be manufactured in a sterile facility. Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all. Stability Testing Time Points.
From arctutorials.com
Stability Testing Complete Tutorial ARC Tutorials Stability Testing Time Points Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. The purpose of stability testing is to provide evidence on how. Stability Testing Time Points.
From u-tor.com
What is Stability Testing? A Practical Guide with Example UTOR Stability Testing Time Points This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. The purpose of stability testing is to provide evidence on how the quality. Stability Testing Time Points.
From www.slideshare.net
ICH Guidelines for stability testing Stability Testing Time Points The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. The purpose of stability testing is to provide. Stability Testing Time Points.
From www.youtube.com
Jury Stability Test SecondOrder DiscreteTime System Calculations Stability Testing Time Points This guideline is intended to provide recommendations on how to use stability data generated in accordance with the principles. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. The purpose of stability testing is to provide evidence on how the quality of a drug. Stability Testing Time Points.
From www.researchgate.net
Stability test of S + NSnO2STS photocatalyst by value over time on Stability Testing Time Points This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. The purpose of stability testing is to provide evidence on how the quality. Stability Testing Time Points.
From u-tor.com
What is Stability Testing? A Practical Guide with Example UTOR Stability Testing Time Points Sterile drug product batches (supporting anda submission) should be manufactured in a sterile facility. Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under. Stability Testing Time Points.
From u-tor.com
What is Stability Testing? A Practical Guide with Example UTOR Stability Testing Time Points This guideline is intended to provide recommendations on how to use stability data generated in accordance with the principles. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the. This document defines the stability data package for a new drug substance or drug product that. Stability Testing Time Points.
From www.researchgate.net
a) Stability test by exerting high shear rate; buffer flowing at 7500 s Stability Testing Time Points Stability schedule such that only samples on the extremes of certain design factors (e.g., strength, container size and/or fill) are tested at all time. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. This guideline is intended to provide recommendations on how. Stability Testing Time Points.