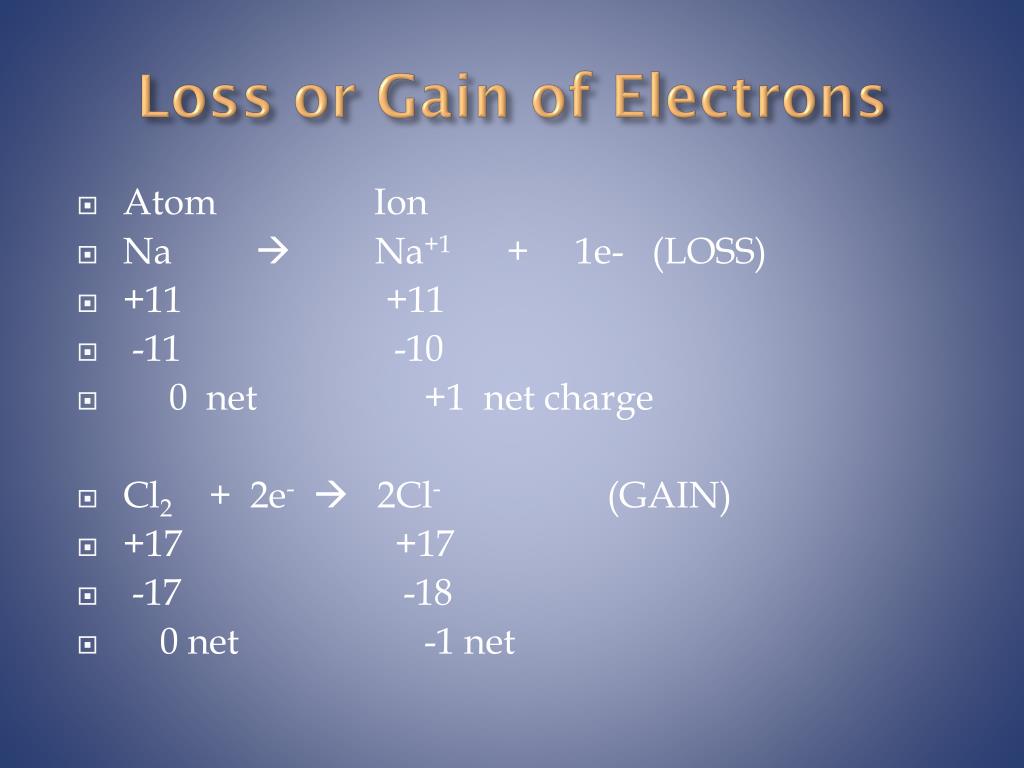
Do Positive Ions Gain Or Lose Electrons . atoms lose electrons from their outer shell when they form positive ions, called. Individual atoms can gain or lose. These ions are positive because they. Ions form when atoms lose or gain. compounds formed from positive and negative ions are called ionic compounds. Ions are atoms with a charge. elements that are metals tend to lose electrons and become positively charged ions called cations. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to. Atoms are neutral, which means there is an equal number of protons and electrons. Or group of atoms with a positive or negative. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative.
from www.slideserve.com
compounds formed from positive and negative ions are called ionic compounds. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to. Or group of atoms with a positive or negative. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. atoms lose electrons from their outer shell when they form positive ions, called. Atoms are neutral, which means there is an equal number of protons and electrons. Ions form when atoms lose or gain. elements that are metals tend to lose electrons and become positively charged ions called cations. Ions are atoms with a charge. Individual atoms can gain or lose.
PPT History and Progression of Atomic Theory PowerPoint Presentation
Do Positive Ions Gain Or Lose Electrons a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. compounds formed from positive and negative ions are called ionic compounds. Atoms are neutral, which means there is an equal number of protons and electrons. elements that are metals tend to lose electrons and become positively charged ions called cations. Ions are atoms with a charge. Ions form when atoms lose or gain. Or group of atoms with a positive or negative. atoms lose electrons from their outer shell when they form positive ions, called. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to. These ions are positive because they. Individual atoms can gain or lose.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Do Positive Ions Gain Or Lose Electrons atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to. atoms lose electrons from their outer shell when they form positive ions, called. elements that are metals tend to lose electrons and become positively charged ions called cations. Or group of atoms with a positive or. Do Positive Ions Gain Or Lose Electrons.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science Do Positive Ions Gain Or Lose Electrons atoms lose electrons from their outer shell when they form positive ions, called. Ions form when atoms lose or gain. These ions are positive because they. Individual atoms can gain or lose. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Or group. Do Positive Ions Gain Or Lose Electrons.
From www.slideshare.net
Understanding The Chemistry Of Atoms to Ions Do Positive Ions Gain Or Lose Electrons atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to. Ions are atoms with a charge. Atoms are neutral, which means there is an equal number of protons and electrons. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its. Do Positive Ions Gain Or Lose Electrons.
From chem.libretexts.org
3.2 Lewis Dot Diagrams Chemistry LibreTexts Do Positive Ions Gain Or Lose Electrons elements that are metals tend to lose electrons and become positively charged ions called cations. These ions are positive because they. compounds formed from positive and negative ions are called ionic compounds. Ions form when atoms lose or gain. Ions are atoms with a charge. a cation (a positive ion) forms when a neutral atom loses one. Do Positive Ions Gain Or Lose Electrons.
From liliannorman.wordpress.com
Explainer Ions and radicals in our world Education Updates Do Positive Ions Gain Or Lose Electrons compounds formed from positive and negative ions are called ionic compounds. Individual atoms can gain or lose. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Or group of atoms with a positive or negative. Ions are atoms with a charge. when. Do Positive Ions Gain Or Lose Electrons.
From www.youtube.com
Which elements tend to lose electrons? What charge will they Do Positive Ions Gain Or Lose Electrons when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. These ions are positive because they. Ions form when atoms lose or gain. Atoms are neutral, which means there is an equal number of protons and electrons. Individual atoms can gain or lose. a cation (a positive ion) forms. Do Positive Ions Gain Or Lose Electrons.
From www.expii.com
Ions — Definition & Overview Expii Do Positive Ions Gain Or Lose Electrons when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. compounds formed from positive and negative ions are called ionic compounds. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to. Individual atoms can gain or lose.. Do Positive Ions Gain Or Lose Electrons.
From www.mooramo.com
Gaining and Losing Electrons Mooramo Do Positive Ions Gain Or Lose Electrons atoms lose electrons from their outer shell when they form positive ions, called. Atoms are neutral, which means there is an equal number of protons and electrons. elements that are metals tend to lose electrons and become positively charged ions called cations. These ions are positive because they. Ions are atoms with a charge. atoms that lose. Do Positive Ions Gain Or Lose Electrons.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Do Positive Ions Gain Or Lose Electrons Atoms are neutral, which means there is an equal number of protons and electrons. Individual atoms can gain or lose. Ions are atoms with a charge. compounds formed from positive and negative ions are called ionic compounds. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an. Do Positive Ions Gain Or Lose Electrons.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Do Positive Ions Gain Or Lose Electrons atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to. Atoms are neutral, which means there is an equal number of protons and electrons. Individual atoms can gain or lose. compounds formed from positive and negative ions are called ionic compounds. atoms lose electrons from their. Do Positive Ions Gain Or Lose Electrons.
From lifhyscapeuciscel.netlify.app
Br Lose Or Gain Electrons Do Positive Ions Gain Or Lose Electrons compounds formed from positive and negative ions are called ionic compounds. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to. These ions are positive because they. Atoms are neutral, which means there is an equal number of protons and electrons. Ions are atoms with a charge.. Do Positive Ions Gain Or Lose Electrons.
From slideplayer.com
Ions and Ionic Compounds ppt download Do Positive Ions Gain Or Lose Electrons Ions are atoms with a charge. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Ions form when atoms lose or gain. These ions are positive because they. Individual atoms can gain or lose. elements that are metals tend to lose electrons and become positively charged ions called. Do Positive Ions Gain Or Lose Electrons.
From mungfali.com
Ppt Chapter 1 The Chemical Basis Of Life Powerpoint Presentation 7FE Do Positive Ions Gain Or Lose Electrons atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Ions are atoms with a charge. Individual atoms can gain or lose. elements that are metals tend. Do Positive Ions Gain Or Lose Electrons.
From stock.adobe.com
Oxidation and reduction infographic diagram chemistry science education Do Positive Ions Gain Or Lose Electrons Ions are atoms with a charge. elements that are metals tend to lose electrons and become positively charged ions called cations. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its. Do Positive Ions Gain Or Lose Electrons.
From slideplayer.com
The Atoman introduction ppt download Do Positive Ions Gain Or Lose Electrons compounds formed from positive and negative ions are called ionic compounds. Ions form when atoms lose or gain. Atoms are neutral, which means there is an equal number of protons and electrons. Or group of atoms with a positive or negative. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a. Do Positive Ions Gain Or Lose Electrons.
From www.periodictableprintable.com
Periodic Table Elements Lose Or Gain Electrons 2024 Periodic Table Do Positive Ions Gain Or Lose Electrons Ions are atoms with a charge. atoms lose electrons from their outer shell when they form positive ions, called. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to. Or group of atoms with a positive or negative. Ions form when atoms lose or gain. when. Do Positive Ions Gain Or Lose Electrons.
From www.slideserve.com
PPT Structure of An Atom Notes PowerPoint Presentation, free download Do Positive Ions Gain Or Lose Electrons elements that are metals tend to lose electrons and become positively charged ions called cations. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. These ions are positive because they. atoms that lose electrons acquire a positive charge as a result because they are left with fewer. Do Positive Ions Gain Or Lose Electrons.
From www.haryanaexams.in
Positive ions are formed from the neutral atom by the Do Positive Ions Gain Or Lose Electrons compounds formed from positive and negative ions are called ionic compounds. Atoms are neutral, which means there is an equal number of protons and electrons. Ions are atoms with a charge. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to. when forming ions, elements typically. Do Positive Ions Gain Or Lose Electrons.
From studylib.net
By GAINING or LOSING electrons Do Positive Ions Gain Or Lose Electrons atoms lose electrons from their outer shell when they form positive ions, called. elements that are metals tend to lose electrons and become positively charged ions called cations. Atoms are neutral, which means there is an equal number of protons and electrons. Or group of atoms with a positive or negative. a cation (a positive ion) forms. Do Positive Ions Gain Or Lose Electrons.
From www.stonecoldhands.com
Ions Predict Charge Stone Cold Chemistry Talk Ions Predict Charge Do Positive Ions Gain Or Lose Electrons a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. atoms lose electrons from their outer shell when they form positive ions, called. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. These. Do Positive Ions Gain Or Lose Electrons.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Do Positive Ions Gain Or Lose Electrons Ions are atoms with a charge. Ions form when atoms lose or gain. compounds formed from positive and negative ions are called ionic compounds. Atoms are neutral, which means there is an equal number of protons and electrons. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. These. Do Positive Ions Gain Or Lose Electrons.
From projectopenletter.com
Do Metals Form Positive Or Negative Ions Do Positive Ions Gain Or Lose Electrons a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. elements that are metals tend to lose electrons and become positively charged ions called cations. These ions are positive because they. Ions form when atoms lose or gain. Ions are atoms with a charge.. Do Positive Ions Gain Or Lose Electrons.
From www.slideserve.com
PPT 2.2 The Periodic table and Chemical Properties PowerPoint Do Positive Ions Gain Or Lose Electrons Ions are atoms with a charge. atoms lose electrons from their outer shell when they form positive ions, called. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. atoms that lose electrons acquire a positive charge as a result because they are. Do Positive Ions Gain Or Lose Electrons.
From scientifictutor.org
Chem Ions Scientific Tutor Do Positive Ions Gain Or Lose Electrons atoms lose electrons from their outer shell when they form positive ions, called. Ions are atoms with a charge. Or group of atoms with a positive or negative. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. a cation (a positive ion) forms when a neutral atom. Do Positive Ions Gain Or Lose Electrons.
From www.nagwa.com
Question Video Finding the Number of Electrons Lost or Gained by an Do Positive Ions Gain Or Lose Electrons a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. elements that are metals tend to lose electrons and become positively charged ions called cations. Individual atoms can gain or lose. Ions form when atoms lose or gain. These ions are positive because they.. Do Positive Ions Gain Or Lose Electrons.
From www.slideserve.com
PPT Oxidation an atom loses one or more electrons . PowerPoint Do Positive Ions Gain Or Lose Electrons elements that are metals tend to lose electrons and become positively charged ions called cations. Individual atoms can gain or lose. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. when forming ions, elements typically gain or lose the minimum number of. Do Positive Ions Gain Or Lose Electrons.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation, free download ID2508414 Do Positive Ions Gain Or Lose Electrons a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Individual atoms can gain or lose. These ions are positive because they. atoms lose electrons from their outer shell when they form positive ions, called. Or group of atoms with a positive or negative.. Do Positive Ions Gain Or Lose Electrons.
From www.slideserve.com
PPT History and Progression of Atomic Theory PowerPoint Presentation Do Positive Ions Gain Or Lose Electrons Individual atoms can gain or lose. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Ions are atoms with a charge. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Atoms are neutral,. Do Positive Ions Gain Or Lose Electrons.
From www.slideserve.com
PPT Chapter 22 Chemical Bonds PowerPoint Presentation, free Do Positive Ions Gain Or Lose Electrons atoms lose electrons from their outer shell when they form positive ions, called. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to. Individual atoms can gain or lose. elements that are metals tend to lose electrons and become positively charged ions called cations. These ions. Do Positive Ions Gain Or Lose Electrons.
From www.youtube.com
How ions are formed by valence electron loss or gain GCSE CHEMISTRY Do Positive Ions Gain Or Lose Electrons Or group of atoms with a positive or negative. elements that are metals tend to lose electrons and become positively charged ions called cations. Atoms are neutral, which means there is an equal number of protons and electrons. Ions are atoms with a charge. atoms that lose electrons acquire a positive charge as a result because they are. Do Positive Ions Gain Or Lose Electrons.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation, free download ID2508414 Do Positive Ions Gain Or Lose Electrons Individual atoms can gain or lose. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Or group of atoms with a positive or negative. elements that are metals tend to lose electrons and become positively charged ions called cations. Atoms are neutral, which. Do Positive Ions Gain Or Lose Electrons.
From slideplayer.com
VTT 200 General Sciences Chemistry ppt download Do Positive Ions Gain Or Lose Electrons atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to. Ions are atoms with a charge. atoms lose electrons from their outer shell when they form positive ions, called. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its. Do Positive Ions Gain Or Lose Electrons.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.37 Understand How Ions are formed by Electron Do Positive Ions Gain Or Lose Electrons compounds formed from positive and negative ions are called ionic compounds. elements that are metals tend to lose electrons and become positively charged ions called cations. Or group of atoms with a positive or negative. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to. . Do Positive Ions Gain Or Lose Electrons.
From www.slideserve.com
PPT Recap Atomic Structure PowerPoint Presentation, free download Do Positive Ions Gain Or Lose Electrons atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to. Atoms are neutral, which means there is an equal number of protons and electrons. elements that are metals tend to lose electrons and become positively charged ions called cations. Ions form when atoms lose or gain. Or. Do Positive Ions Gain Or Lose Electrons.
From www.shalom-education.com
Isotopes and Ions GCSE Physics Revision Do Positive Ions Gain Or Lose Electrons Ions form when atoms lose or gain. These ions are positive because they. Atoms are neutral, which means there is an equal number of protons and electrons. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. compounds formed from positive and negative ions are called ionic compounds. . Do Positive Ions Gain Or Lose Electrons.