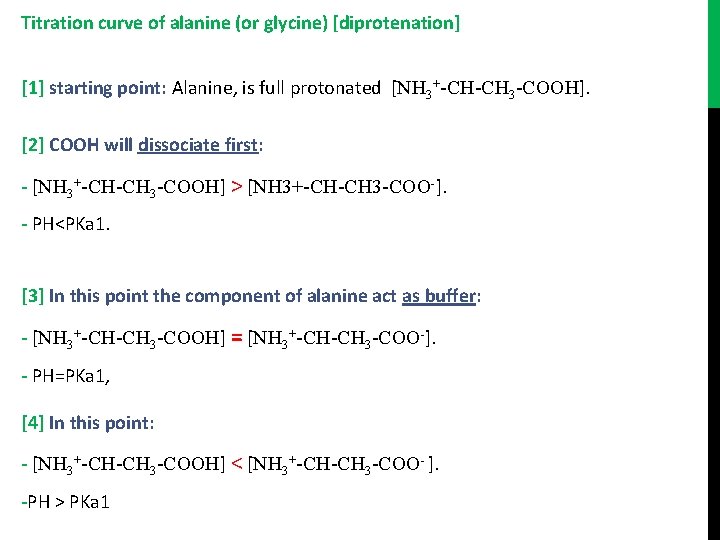
Titration Curve Of Alanine . each leg of the titration curve is calculated separately. each leg of the titration curve is calculated separately. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. The first leg, from ph 1 to 6, corresponds to the dissociation of. the isoelectric points range from 5.5 to 6.2. learn how to estimate the pka values, pi and buffering region of alanine by titrating it with naoh and hcl. Titration curves show the neutralization of these acids by added base, and the change. the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h 2 a +. Titration curves are produced by monitoring the ph of a given volume of a sample solution after successive addition of.
from slidetodoc.com
The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h 2 a +. The first leg, from ph 1 to 6, corresponds to the dissociation of. Titration curves show the neutralization of these acids by added base, and the change. the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. learn how to estimate the pka values, pi and buffering region of alanine by titrating it with naoh and hcl. each leg of the titration curve is calculated separately. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. each leg of the titration curve is calculated separately. the isoelectric points range from 5.5 to 6.2. Titration curves are produced by monitoring the ph of a given volume of a sample solution after successive addition of.
Titration curve of amino acids BCH 312 PRACTICAL
Titration Curve Of Alanine Titration curves show the neutralization of these acids by added base, and the change. the isoelectric points range from 5.5 to 6.2. The first leg, from ph 1 to 6, corresponds to the dissociation of. Titration curves are produced by monitoring the ph of a given volume of a sample solution after successive addition of. each leg of the titration curve is calculated separately. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h 2 a +. learn how to estimate the pka values, pi and buffering region of alanine by titrating it with naoh and hcl. Titration curves show the neutralization of these acids by added base, and the change. each leg of the titration curve is calculated separately. the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship.
From www.researchgate.net
Potentiometric titration curves of the DETalanine system Download Titration Curve Of Alanine The first leg, from ph 1 to 6, corresponds to the dissociation of. The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h 2 a +. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. Titration curves are produced by monitoring the ph of a given volume of. Titration Curve Of Alanine.
From www.chegg.com
Solved . The following diagram depicts the titration curve Titration Curve Of Alanine each leg of the titration curve is calculated separately. the isoelectric points range from 5.5 to 6.2. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h 2 a +. learn how to estimate the pka. Titration Curve Of Alanine.
From www.researchgate.net
NMR titration curve of NacetylLalanine 29 with the dicationic Titration Curve Of Alanine At a ph lower than 2, both the carboxylate and amine functions are protonated, so. The first leg, from ph 1 to 6, corresponds to the dissociation of. the isoelectric points range from 5.5 to 6.2. the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. The first leg, from ph 1 to 6, corresponds to the. Titration Curve Of Alanine.
From www.researchgate.net
6 Titration curve of alanine (monoamino and monocarboxylic acid). A Titration Curve Of Alanine Titration curves are produced by monitoring the ph of a given volume of a sample solution after successive addition of. Titration curves show the neutralization of these acids by added base, and the change. The first leg, from ph 1 to 6, corresponds to the dissociation of. The first leg, from ph 1 to 6, corresponds to the dissociation of. Titration Curve Of Alanine.
From mavink.com
Protein Titration Curve Titration Curve Of Alanine the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. learn how to estimate the pka values, pi and buffering region of alanine by titrating it with naoh and hcl. The first leg, from ph 1 to 6, corresponds to the dissociation of. Titration curves show the neutralization of these acids by added base, and the change.. Titration Curve Of Alanine.
From cwsimons.com
How to Draw Titration Curves of Amino Acids Food Science Toolbox Titration Curve Of Alanine each leg of the titration curve is calculated separately. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. The first leg, from ph 1 to 6, corresponds to the dissociation of. each leg of the titration curve is calculated separately. Titration curves show the neutralization of these acids by added base, and. Titration Curve Of Alanine.
From www.numerade.com
SOLVED For titration of alanine with 0.1 M NaOH in the presence and Titration Curve Of Alanine The first leg, from ph 1 to 6, corresponds to the dissociation of. Titration curves are produced by monitoring the ph of a given volume of a sample solution after successive addition of. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. each leg of the titration curve is calculated separately. each. Titration Curve Of Alanine.
From www.slideserve.com
PPT Chemical Nature of the Amino Acids PowerPoint Presentation ID16008 Titration Curve Of Alanine each leg of the titration curve is calculated separately. Titration curves are produced by monitoring the ph of a given volume of a sample solution after successive addition of. learn how to estimate the pka values, pi and buffering region of alanine by titrating it with naoh and hcl. At a ph lower than 2, both the carboxylate. Titration Curve Of Alanine.
From www.researchgate.net
Potentiometric titration curves of the DETalanine system Download Titration Curve Of Alanine the isoelectric points range from 5.5 to 6.2. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. each leg of the titration curve is calculated separately. Titration curves show the neutralization of these acids by added base, and the change. The first leg, from ph 1 to 6, corresponds to the dissociation. Titration Curve Of Alanine.
From slideplayer.com
Titration curve of amino acids ppt download Titration Curve Of Alanine the isoelectric points range from 5.5 to 6.2. the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. Titration curves show the neutralization of these acids by added base, and the change. each leg of the titration curve is calculated separately. learn how to estimate the pka values, pi and buffering region of alanine by. Titration Curve Of Alanine.
From chempedia.info
Titration curve, alanine Big Chemical Encyclopedia Titration Curve Of Alanine each leg of the titration curve is calculated separately. Titration curves show the neutralization of these acids by added base, and the change. the isoelectric points range from 5.5 to 6.2. The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h 2 a +. At a ph lower than 2, both the. Titration Curve Of Alanine.
From www.numerade.com
SOLVEDA titration of an acidified solution of alanine gives the Titration Curve Of Alanine learn how to estimate the pka values, pi and buffering region of alanine by titrating it with naoh and hcl. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. the isoelectric points range from 5.5 to 6.2. The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine,. Titration Curve Of Alanine.
From chempedia.info
Alanine protonation Big Chemical Encyclopedia Titration Curve Of Alanine the isoelectric points range from 5.5 to 6.2. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. each leg of the titration curve is calculated separately. Titration curves show the neutralization of these acids by added base, and the change. the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship.. Titration Curve Of Alanine.
From www.chegg.com
Consider the amino acid alanine (Figure 3) and the Titration Curve Of Alanine the isoelectric points range from 5.5 to 6.2. The first leg, from ph 1 to 6, corresponds to the dissociation of. the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. Titration curves are produced by monitoring the ph of a given volume of a sample solution after successive addition of. At a ph lower than 2,. Titration Curve Of Alanine.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID1205383 Titration Curve Of Alanine each leg of the titration curve is calculated separately. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. Titration curves show the neutralization of these acids by added base, and the change. The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h 2 a +. The first. Titration Curve Of Alanine.
From www.chegg.com
Solved 5) 5 The following is a titration curve of alanine. Titration Curve Of Alanine The first leg, from ph 1 to 6, corresponds to the dissociation of. learn how to estimate the pka values, pi and buffering region of alanine by titrating it with naoh and hcl. Titration curves show the neutralization of these acids by added base, and the change. Titration curves are produced by monitoring the ph of a given volume. Titration Curve Of Alanine.
From www.slideserve.com
PPT Amino Acids and Peptides PowerPoint Presentation, free download Titration Curve Of Alanine each leg of the titration curve is calculated separately. Titration curves are produced by monitoring the ph of a given volume of a sample solution after successive addition of. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. the isoelectric points range from 5.5 to 6.2. The first leg, from ph 1. Titration Curve Of Alanine.
From www.slideserve.com
PPT Biochemistry PowerPoint Presentation, free download ID5880126 Titration Curve Of Alanine each leg of the titration curve is calculated separately. The first leg, from ph 1 to 6, corresponds to the dissociation of. the isoelectric points range from 5.5 to 6.2. The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h 2 a +. each leg of the titration curve is calculated. Titration Curve Of Alanine.
From www.youtube.com
ALANINE TITRATION CURVE in simple way🤓 YouTube Titration Curve Of Alanine the isoelectric points range from 5.5 to 6.2. The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h 2 a +. each leg of the titration curve is calculated separately. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. the titration curve for alanine in. Titration Curve Of Alanine.
From slidetodoc.com
Titration curve of amino acids BCH 312 PRACTICAL Titration Curve Of Alanine the isoelectric points range from 5.5 to 6.2. The first leg, from ph 1 to 6, corresponds to the dissociation of. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. each leg of the titration curve is calculated separately. the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. The. Titration Curve Of Alanine.
From www.slideserve.com
PPT Amino Acids and the Primary Structure of Proteins PowerPoint Titration Curve Of Alanine each leg of the titration curve is calculated separately. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. the isoelectric points range from 5.5 to 6.2. Titration curves are produced by monitoring the ph of a given volume of a sample solution after successive addition of. The first leg, from ph 1. Titration Curve Of Alanine.
From animalia-life.club
Titration Curve Amino Acid Titration Curve Of Alanine Titration curves are produced by monitoring the ph of a given volume of a sample solution after successive addition of. Titration curves show the neutralization of these acids by added base, and the change. each leg of the titration curve is calculated separately. The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h. Titration Curve Of Alanine.
From www.animalia-life.club
Titration Curve Amino Acid Titration Curve Of Alanine each leg of the titration curve is calculated separately. the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. learn how to estimate the pka values, pi and buffering region of alanine by titrating it with naoh and hcl. the isoelectric points range from 5.5 to 6.2. each leg of the titration curve is. Titration Curve Of Alanine.
From www.chegg.com
Solved The figure below shows a titration of the amino acid Titration Curve Of Alanine The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h 2 a +. each leg of the titration curve is calculated separately. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. the isoelectric points range from 5.5 to 6.2. learn how to estimate the pka. Titration Curve Of Alanine.
From www.researchgate.net
NMR titration curve of NacetylLalanine 29 with the triscationic Titration Curve Of Alanine The first leg, from ph 1 to 6, corresponds to the dissociation of. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. Titration curves show the neutralization of these acids by added base, and the change. learn how to estimate the pka values, pi and buffering region of alanine by titrating it with. Titration Curve Of Alanine.
From www.researchgate.net
Potentiometric titration curves of the DETalanine system Download Titration Curve Of Alanine each leg of the titration curve is calculated separately. the isoelectric points range from 5.5 to 6.2. the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. each leg of the titration curve is calculated separately. The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h 2 a +.. Titration Curve Of Alanine.
From www.researchgate.net
Potentiometric titration curves of the DETalanine system Download Titration Curve Of Alanine learn how to estimate the pka values, pi and buffering region of alanine by titrating it with naoh and hcl. At a ph lower than 2, both the carboxylate and amine functions are protonated, so. the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. each leg of the titration curve is calculated separately. The first. Titration Curve Of Alanine.
From www.chegg.com
Solved [1 mark] The titration curve of ALANINE is shown Titration Curve Of Alanine The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h 2 a +. learn how to estimate the pka values, pi and buffering region of alanine by titrating it with naoh and hcl. each leg of the titration curve is calculated separately. Titration curves are produced by monitoring the ph of a. Titration Curve Of Alanine.
From www.chegg.com
Solved The titration curve of alanine shows the ionization Titration Curve Of Alanine The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h 2 a +. the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. learn how to estimate the pka values, pi and buffering region of alanine by titrating it with naoh and hcl. each leg of the titration curve is. Titration Curve Of Alanine.
From www.chegg.com
Solved 6. For the following titration curve of alanine, what Titration Curve Of Alanine the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. Titration curves are produced by monitoring the ph of a given volume of a sample solution after successive addition of. the isoelectric points range from 5.5 to 6.2. learn how to estimate the pka values, pi and buffering region of alanine by titrating it with naoh. Titration Curve Of Alanine.
From www.researchgate.net
Calibration curve for alanine in the range 0.02 g/mL and 1 g/mL. The Titration Curve Of Alanine the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. each leg of the titration curve is calculated separately. The first leg, from ph 1 to 6, corresponds to the dissociation of. each leg of the titration curve is calculated separately. The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine,. Titration Curve Of Alanine.
From www.researchgate.net
6 Titration curve of alanine (monoamino and monocarboxylic acid). A Titration Curve Of Alanine the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h 2 a +. the isoelectric points range from 5.5 to 6.2. each leg of the titration curve is calculated separately. Titration curves are produced by monitoring the ph of a. Titration Curve Of Alanine.
From www.chegg.com
Solved 4. (2 points) Consider alanine titration curve below. Titration Curve Of Alanine Titration curves show the neutralization of these acids by added base, and the change. The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h 2 a +. the isoelectric points range from 5.5 to 6.2. learn how to estimate the pka values, pi and buffering region of alanine by titrating it with. Titration Curve Of Alanine.
From www.researchgate.net
Figure S2 Calibration curve of labeled alanine. The 20 natural amino Titration Curve Of Alanine learn how to estimate the pka values, pi and buffering region of alanine by titrating it with naoh and hcl. each leg of the titration curve is calculated separately. the titration curve for alanine in figure \(\pageindex{2}\) demonstrates this relationship. The first leg, from ph 1 to 6, corresponds to the dissociation of protonated alanine, h 2. Titration Curve Of Alanine.
From www.youtube.com
Ch.3 Amino Acid part 4 Titration curve of non polar amino acid Titration Curve Of Alanine Titration curves are produced by monitoring the ph of a given volume of a sample solution after successive addition of. the isoelectric points range from 5.5 to 6.2. each leg of the titration curve is calculated separately. The first leg, from ph 1 to 6, corresponds to the dissociation of. each leg of the titration curve is. Titration Curve Of Alanine.