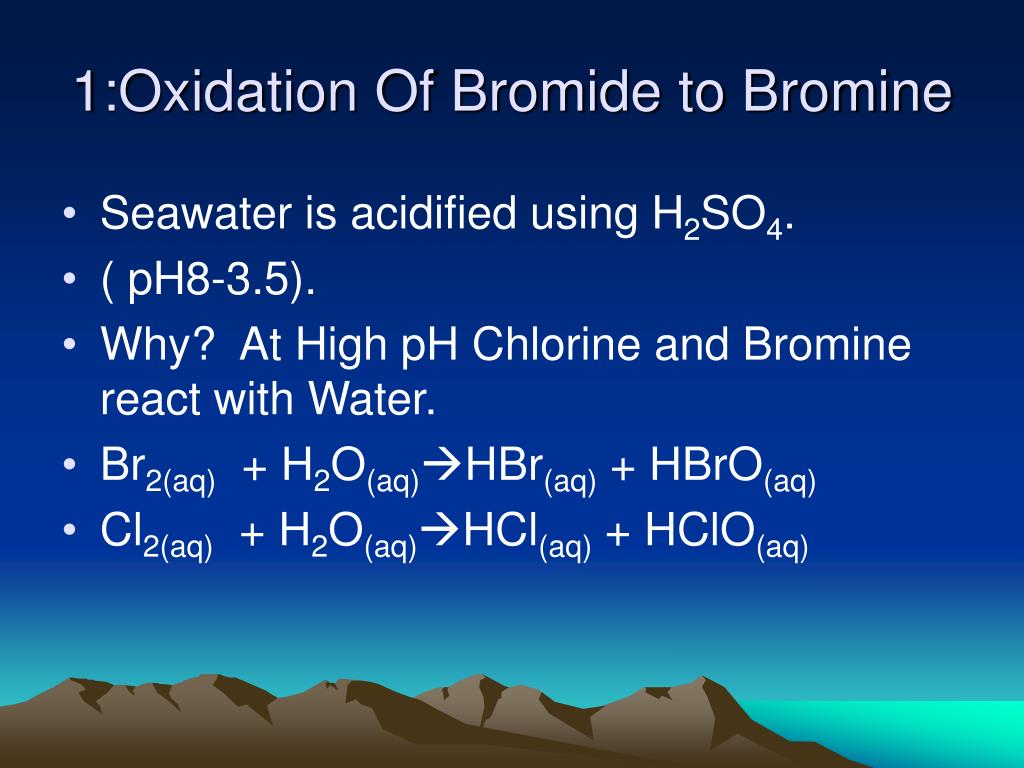
Bromide Ion Oxidation . For example, chlorine can oxidise the bromide ions (in, for. The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: In each case, a halogen higher in the group can oxidise the ions of one lower down. There is one cobalt ion. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a.
from www.slideserve.com
In each case, a halogen higher in the group can oxidise the ions of one lower down. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a. There is one cobalt ion. For example, chlorine can oxidise the bromide ions (in, for.
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation
Bromide Ion Oxidation In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. For example, chlorine can oxidise the bromide ions (in, for. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. There is one cobalt ion. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a. In each case, a halogen higher in the group can oxidise the ions of one lower down. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. The commercial preparation of bromine involves the oxidation of bromide ion by chlorine:
From slideplayer.com
Bromide Photooxidation Sensitized to Visible Light in Consecutive Ion Bromide Ion Oxidation For example, chlorine can oxidise the bromide ions (in, for. In each case, a halogen higher in the group can oxidise the ions of one lower down. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. 2 br − (a q) + cl 2 (g). Bromide Ion Oxidation.
From www.researchgate.net
Plausible mechanism of bromide oxidation by complex (1) in presence of Bromide Ion Oxidation For example, chlorine can oxidise the bromide ions (in, for. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. In each case, a halogen higher in the group can. Bromide Ion Oxidation.
From www.youtube.com
Quick video Balancing an oxidation reduction reaction in base [bromine Bromide Ion Oxidation In each case, a halogen higher in the group can oxidise the ions of one lower down. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a. The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: In the aqueous bulk, oxidation of bromide by ozone. Bromide Ion Oxidation.
From www.docsity.com
Oxidation of Bromide Ions by Hydrogen Peroxide in Aqueous Solutions Bromide Ion Oxidation Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. For example, chlorine can oxidise the bromide ions (in, for. 2 br − (a q) + cl 2 (g) br. Bromide Ion Oxidation.
From dokumen.tips
(PDF) Mechanism of bromide ion oxidation on Pt electrode in CH2Cl2 Bromide Ion Oxidation In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. There is one cobalt ion. In each case, a halogen higher in the group can oxidise the ions of one lower down. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a. For. Bromide Ion Oxidation.
From www.doubtnut.com
Bromine is commercially produced by the oxidation of bromide ions in n Bromide Ion Oxidation In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. In each case, a halogen higher in the group can oxidise the ions of one lower down. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. There is one. Bromide Ion Oxidation.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Bromide Ion Oxidation In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. There is one cobalt ion. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a. For example, chlorine can oxidise the bromide ions (in, for. Based on the ion chromatography and lsv analysis,. Bromide Ion Oxidation.
From www.shutterstock.com
Sodium Bromide Properties Chemical Compound Structure Stock Vector Bromide Ion Oxidation In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a. There is one cobalt ion. In each case, a halogen. Bromide Ion Oxidation.
From www.science-revision.co.uk
Electrolysis Bromide Ion Oxidation For example, chlorine can oxidise the bromide ions (in, for. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a. There is one cobalt ion. The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: In each case, a halogen higher in the group can oxidise. Bromide Ion Oxidation.
From www.chegg.com
Solved Bromide ion is oxidized by bromate ion in acidic Bromide Ion Oxidation There is one cobalt ion. The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a. For example, chlorine can oxidise the bromide ions (in, for. In each case, a halogen higher in the group can oxidise. Bromide Ion Oxidation.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Bromide Ion Oxidation In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. In each case, a halogen higher in the group can oxidise the ions of one lower down. The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: 2 br − (a q) + cl 2 (g) br 2 (l) + 2. Bromide Ion Oxidation.
From www.researchgate.net
(PDF) Radiation chemical oxidation of bromide ions and formation of Bromide Ion Oxidation The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: For example, chlorine can oxidise the bromide ions (in, for. In each case, a halogen higher in the group can oxidise the ions of one lower down. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. There is one cobalt. Bromide Ion Oxidation.
From www.toppr.com
The stoichiometric equation for the oxidation of bromide ions by Bromide Ion Oxidation In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: For example, chlorine can oxidise the bromide. Bromide Ion Oxidation.
From www.chegg.com
Solved 9. b) The oxidation of bromide ions by bromate in Bromide Ion Oxidation For example, chlorine can oxidise the bromide ions (in, for. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a. In each case, a halogen. Bromide Ion Oxidation.
From www.chegg.com
Solved Bromide ion is oxidized by bromate ion in acidic Bromide Ion Oxidation Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. For example, chlorine can oxidise the bromide ions (in, for. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. 2 br − (a q) + cl 2 (g) br. Bromide Ion Oxidation.
From www.toppr.com
The stoichiometric equation for the oxidation of bromide ions by Bromide Ion Oxidation Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a. For example,. Bromide Ion Oxidation.
From www.numerade.com
bromide ion is oxidized by bromate ion in acidic solution 5br aq bro3 Bromide Ion Oxidation In each case, a halogen higher in the group can oxidise the ions of one lower down. There is one cobalt ion. The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. 2 br − (a q) + cl 2 (g). Bromide Ion Oxidation.
From www.chegg.com
Solved The oxidation of bromide ion by persulfate is given Bromide Ion Oxidation Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. In each case, a halogen higher in. Bromide Ion Oxidation.
From www.slideshare.net
Oxidation & reduction Bromide Ion Oxidation In each case, a halogen higher in the group can oxidise the ions of one lower down. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. For example, chlorine. Bromide Ion Oxidation.
From www.toppr.com
The stoichiometric equation for the oxidation of bromide ions by Bromide Ion Oxidation 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. In each case, a halogen higher in the group can oxidise the ions of one lower down. For example, chlorine can oxidise the. Bromide Ion Oxidation.
From www.doubtnut.com
The stoichiometric equation for the oxidation of bromide ions by hydro Bromide Ion Oxidation In each case, a halogen higher in the group can oxidise the ions of one lower down. For example, chlorine can oxidise the bromide ions (in, for. The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. There is one cobalt. Bromide Ion Oxidation.
From www.chegg.com
Solved Assuming the oxidation of bromide ions by bromate in Bromide Ion Oxidation The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: There is one cobalt ion. For example, chlorine can oxidise the bromide ions (in, for. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. In the aqueous bulk, oxidation of bromide by. Bromide Ion Oxidation.
From slideplayer.com
Bromide Photooxidation Sensitized to Visible Light in Consecutive Ion Bromide Ion Oxidation Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. For example, chlorine can oxidise the bromide ions (in, for. In each case, a halogen higher in the group can oxidise the ions of one lower down. 2 br − (a q) + cl 2 (g). Bromide Ion Oxidation.
From byjus.com
Mechanism of Hoffman bromide degradation reaction Bromide Ion Oxidation There is one cobalt ion. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. For example, chlorine can oxidise the bromide ions (in, for. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. The commercial preparation of bromine. Bromide Ion Oxidation.
From blogs.rsc.org
Bromine and Oxygen Redox Species Mediated Highly Selective Electro Bromide Ion Oxidation There is one cobalt ion. For example, chlorine can oxidise the bromide ions (in, for. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of. Bromide Ion Oxidation.
From www.slideshare.net
Oxidation & reduction Bromide Ion Oxidation 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. For example, chlorine can oxidise the bromide ions (in, for. In the aqueous bulk, oxidation. Bromide Ion Oxidation.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Bromide Ion Oxidation For example, chlorine can oxidise the bromide ions (in, for. The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis. Bromide Ion Oxidation.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Bromide Ion Oxidation In each case, a halogen higher in the group can oxidise the ions of one lower down. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. There is one cobalt ion. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex. Bromide Ion Oxidation.
From www.nagwa.com
Question Video Determining Which Ions Are Oxidized and Reduced in the Bromide Ion Oxidation In each case, a halogen higher in the group can oxidise the ions of one lower down. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. For example, chlorine can oxidise the bromide ions (in, for. There is one cobalt ion. 2 br − (a. Bromide Ion Oxidation.
From www.researchgate.net
Spectral changes during bromide ions oxidation by cerium(IV) in Bromide Ion Oxidation The commercial preparation of bromine involves the oxidation of bromide ion by chlorine: Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. For example, chlorine can oxidise the bromide ions (in, for. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −]. Bromide Ion Oxidation.
From www.chegg.com
Solved The oxidation of bromide ions is thought to occur by Bromide Ion Oxidation For example, chlorine can oxidise the bromide ions (in, for. In each case, a halogen higher in the group can oxidise the ions of one lower down. There is one cobalt ion. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Based on the ion chromatography and lsv analysis, two mechanisms were proposed. Bromide Ion Oxidation.
From www.researchgate.net
Tafel plot of bromide ion oxidation at CNTs electrodes in the Bromide Ion Oxidation For example, chlorine can oxidise the bromide ions (in, for. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for. Bromide Ion Oxidation.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation Bromide Ion Oxidation There is one cobalt ion. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. The commercial preparation of bromine involves the oxidation of bromide. Bromide Ion Oxidation.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromide Ion Oxidation For example, chlorine can oxidise the bromide ions (in, for. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Based on the ion chromatography and lsv analysis, two mechanisms were proposed for the formation of reactive bromide species during electrolysis of either organic. 2 br − (a q) + cl 2 (g) br. Bromide Ion Oxidation.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Bromide Ion Oxidation In each case, a halogen higher in the group can oxidise the ions of one lower down. There is one cobalt ion. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. The. Bromide Ion Oxidation.