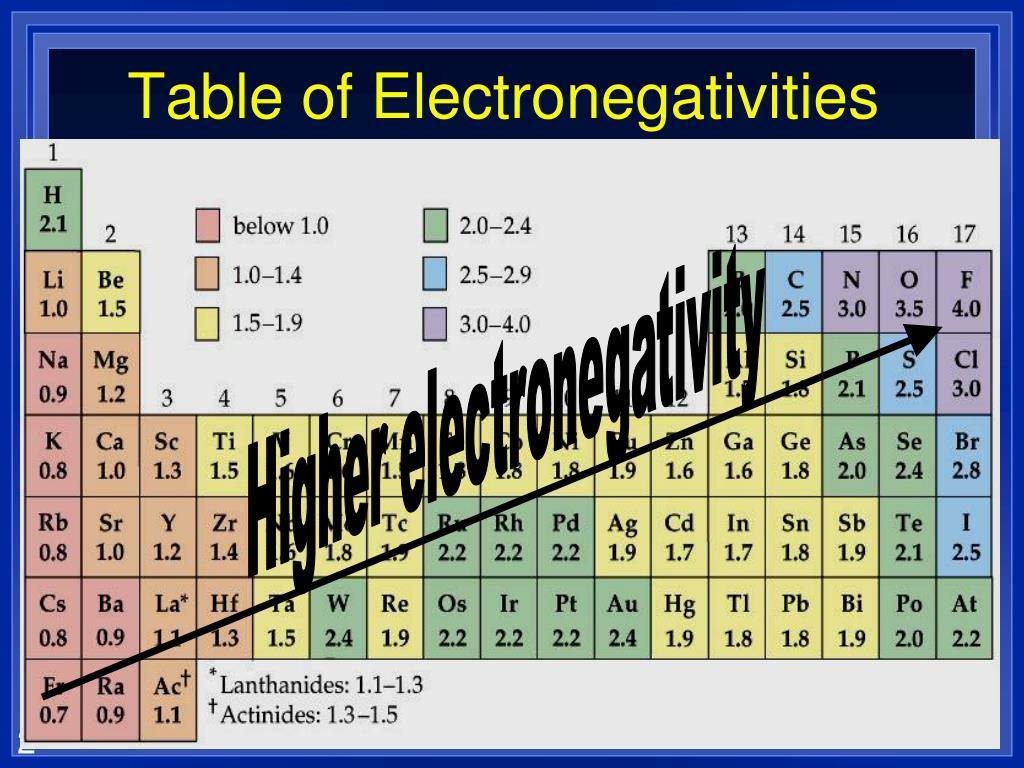
Does Boron Have A High Electronegativity . With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence electrons. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Instead, boron forms unique and intricate structures that Values for electronegativity run from 0 to 4. It can also be used to predict if the resulting molecule will be polar or nonpolar. When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on. The first scale of electronegativity. The electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule.
from www.slideserve.com
It can also be used to predict if the resulting molecule will be polar or nonpolar. Instead, boron forms unique and intricate structures that Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence electrons. Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on. The electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The first scale of electronegativity. Values for electronegativity run from 0 to 4.
PPT Electronegativity? PowerPoint Presentation, free download ID
Does Boron Have A High Electronegativity It can also be used to predict if the resulting molecule will be polar or nonpolar. It can also be used to predict if the resulting molecule will be polar or nonpolar. Values for electronegativity run from 0 to 4. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The first scale of electronegativity. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence electrons. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on. The electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. Instead, boron forms unique and intricate structures that
From www.dreamstime.com
Boron Chemical Element with First Ionization Energy, Atomic Mass and Does Boron Have A High Electronegativity It can also be used to predict if the resulting molecule will be polar or nonpolar. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence electrons. Values for electronegativity run from 0 to 4. Instead, boron forms unique and intricate structures that Elements with high. Does Boron Have A High Electronegativity.
From mungfali.com
Atom Electronegativity Chart Does Boron Have A High Electronegativity 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Instead, boron forms unique and intricate structures. Does Boron Have A High Electronegativity.
From exocoyzqy.blob.core.windows.net
Does Chlorine Have A High Electronegativity at Gordon Maxwell blog Does Boron Have A High Electronegativity When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on. The first scale of electronegativity. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not. Does Boron Have A High Electronegativity.
From www.youtube.com
Electronegativity, Basic Introduction, Periodic Trends Which Element Does Boron Have A High Electronegativity The electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. Values for electronegativity run from 0 to 4. When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. It can also be used to predict if the resulting molecule will be polar or. Does Boron Have A High Electronegativity.
From www.slideserve.com
PPT Electronegativity? PowerPoint Presentation, free download ID Does Boron Have A High Electronegativity Instead, boron forms unique and intricate structures that Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on. When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. Values for electronegativity run from 0 to 4. The first scale of electronegativity. 119. Does Boron Have A High Electronegativity.
From slideplayer.com
Electronegativity and Polarity ppt download Does Boron Have A High Electronegativity Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on. The first scale of electronegativity. When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron. Does Boron Have A High Electronegativity.
From www.youtube.com
Electronegativity ¦ Chemistry of life ¦ Biology ¦ Khan Academy hd YouTube Does Boron Have A High Electronegativity Values for electronegativity run from 0 to 4. The electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence electrons. The first scale of electronegativity. It can also be. Does Boron Have A High Electronegativity.
From chamotgallery.com
How many protons, neutrons and electrons does boron have? (2022) Does Boron Have A High Electronegativity When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. The first scale of electronegativity. It can also be used to predict if the resulting molecule will be polar or nonpolar. Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on. With. Does Boron Have A High Electronegativity.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Does Boron Have A High Electronegativity When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It can also be used to predict. Does Boron Have A High Electronegativity.
From www.numerade.com
SOLVED Arrange the following elements in order of increasing Does Boron Have A High Electronegativity Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on. The electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. The first scale of electronegativity. Instead, boron forms unique and intricate structures that Electronegativity is used to predict whether a bond between atoms. Does Boron Have A High Electronegativity.
From www.sliderbase.com
Pauling scale of electronegativity; Does Boron Have A High Electronegativity The electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. It can also be used to predict if the resulting molecule will be polar or nonpolar. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence electrons. 119. Does Boron Have A High Electronegativity.
From exocoyzqy.blob.core.windows.net
Does Chlorine Have A High Electronegativity at Gordon Maxwell blog Does Boron Have A High Electronegativity Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. Instead, boron forms. Does Boron Have A High Electronegativity.
From www.geeksforgeeks.org
Electronegativity Definition, Meaning, Periodic Trends, Examples Does Boron Have A High Electronegativity Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The first scale of electronegativity. Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Values for. Does Boron Have A High Electronegativity.
From worksheetlistci.z21.web.core.windows.net
Lesson 7.5 Electronegativity And Polarity Does Boron Have A High Electronegativity The electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. Instead, boron forms unique and intricate structures that 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron. Does Boron Have A High Electronegativity.
From iperiodictable.com
How Many Valence Electrons Does Boron Have Periodic Table Element Does Boron Have A High Electronegativity The first scale of electronegativity. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Values for electronegativity run from 0 to 4. It can also be used to predict if the resulting molecule will be polar or nonpolar. Elements with high ionization energies have high electronegativities due to the strong pull exerted by the. Does Boron Have A High Electronegativity.
From chem.libretexts.org
8.1.2 Electronegativity increases and radius decreases towards the Does Boron Have A High Electronegativity It can also be used to predict if the resulting molecule will be polar or nonpolar. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. Values for electronegativity run from 0. Does Boron Have A High Electronegativity.
From exocoyzqy.blob.core.windows.net
Does Chlorine Have A High Electronegativity at Gordon Maxwell blog Does Boron Have A High Electronegativity Values for electronegativity run from 0 to 4. The first scale of electronegativity. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Instead, boron forms unique and intricate structures that When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. It. Does Boron Have A High Electronegativity.
From valenceelectrons.com
How Many Valence Electrons Does Boron (B) Have? Does Boron Have A High Electronegativity When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. It can also be used to predict if the resulting molecule will be polar or nonpolar. Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on. Electronegativity is used to predict whether. Does Boron Have A High Electronegativity.
From utedzz.blogspot.com
Periodic Table Boron Atom Periodic Table Timeline Does Boron Have A High Electronegativity The first scale of electronegativity. The electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Instead, boron forms unique and intricate structures that Elements with high ionization energies have high electronegativities due to the strong pull exerted. Does Boron Have A High Electronegativity.
From www.numerade.com
SOLVED Utc Ihe Re (ctenccs pcetd Imnpurtintemiued What Arrange the Does Boron Have A High Electronegativity With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence electrons. The electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. It can also be used to predict if the resulting molecule will be polar or nonpolar. 119. Does Boron Have A High Electronegativity.
From www.vrogue.co
Printable Electronegativity Periodic Table Electroneg vrogue.co Does Boron Have A High Electronegativity It can also be used to predict if the resulting molecule will be polar or nonpolar. The electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. Instead, boron forms unique and intricate structures that When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of. Does Boron Have A High Electronegativity.
From www.youtube.com
How many valence electrons does boron have?How to find the valence Does Boron Have A High Electronegativity 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized. Does Boron Have A High Electronegativity.
From webgiasi.vn
Electronegativity \u0026 electron affinity Lesson 4 Does Boron Have A High Electronegativity Values for electronegativity run from 0 to 4. When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence electrons. Instead, boron forms unique and intricate structures that. Does Boron Have A High Electronegativity.
From sciencenotes.org
Electronegativity Definition and Trend Does Boron Have A High Electronegativity It can also be used to predict if the resulting molecule will be polar or nonpolar. Values for electronegativity run from 0 to 4. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Instead, boron. Does Boron Have A High Electronegativity.
From thechemistrynotes.com
Difference Between Electronegativity and Electron affinity Does Boron Have A High Electronegativity Values for electronegativity run from 0 to 4. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The first scale of electronegativity. Instead, boron forms unique and intricate structures that The electronegativity depends upon a. Does Boron Have A High Electronegativity.
From www.geeksforgeeks.org
Electronegativity Definition, Meaning, Periodic Trends, Examples Does Boron Have A High Electronegativity When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. The first scale of electronegativity. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence electrons. Elements with high ionization energies have high electronegativities due to the. Does Boron Have A High Electronegativity.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Does Boron Have A High Electronegativity 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Instead, boron forms unique and intricate structures that Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on. It can also be used to predict if the resulting molecule will be polar. Does Boron Have A High Electronegativity.
From chamotgallery.com
How many protons, neutrons and electrons does boron have? (2022) Does Boron Have A High Electronegativity 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. Instead, boron forms unique and intricate structures that Values for electronegativity run from 0 to 4. It can also be used to predict. Does Boron Have A High Electronegativity.
From blograng.com
Top 15 how many neutrons does boron have 2022 Does Boron Have A High Electronegativity When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. The electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. Instead, boron forms unique and intricate structures that Electronegativity is used to predict whether a bond between atoms will be ionic or covalent.. Does Boron Have A High Electronegativity.
From www.nuclear-power.com
Boron Electron Affinity Electronegativity Ionization Energy of Does Boron Have A High Electronegativity The first scale of electronegativity. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Values for electronegativity run from 0 to 4. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence electrons. Instead, boron forms unique and intricate structures. Does Boron Have A High Electronegativity.
From ar.inspiredpencil.com
Periodic Table With Electronegativity And Atomic Radius Does Boron Have A High Electronegativity Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Values for electronegativity run from 0 to 4. It can also be used to predict if the resulting molecule will be polar or nonpolar. The first scale of electronegativity. Elements with high ionization energies have high electronegativities due to the strong pull exerted by the. Does Boron Have A High Electronegativity.
From nl.dreamstime.com
Electronegativity Periodieke Lijst Stock Illustratie Illustration of Does Boron Have A High Electronegativity 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with. Does Boron Have A High Electronegativity.
From www.bigstockphoto.com
Electronegativity Vector & Photo (Free Trial) Bigstock Does Boron Have A High Electronegativity With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence electrons. It can also be used to predict if the resulting molecule will be polar or nonpolar. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself.. Does Boron Have A High Electronegativity.
From secrets-of-periodic-table.blogspot.com
PERIODIC TABLE ELECTRONEGATIVITY NOBLE GASES PAULING Does Boron Have A High Electronegativity Values for electronegativity run from 0 to 4. 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. The first scale of electronegativity. When you think about electronegativity, think of it as a. Does Boron Have A High Electronegativity.
From slideplayer.com
Electronegativity and Polarity ppt download Does Boron Have A High Electronegativity When you think about electronegativity, think of it as a relative value, meaning in comparison to atoms of other. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It can also be used to predict if the resulting molecule will be polar or nonpolar. 119 rows electronegativity is a chemical property which describes how. Does Boron Have A High Electronegativity.