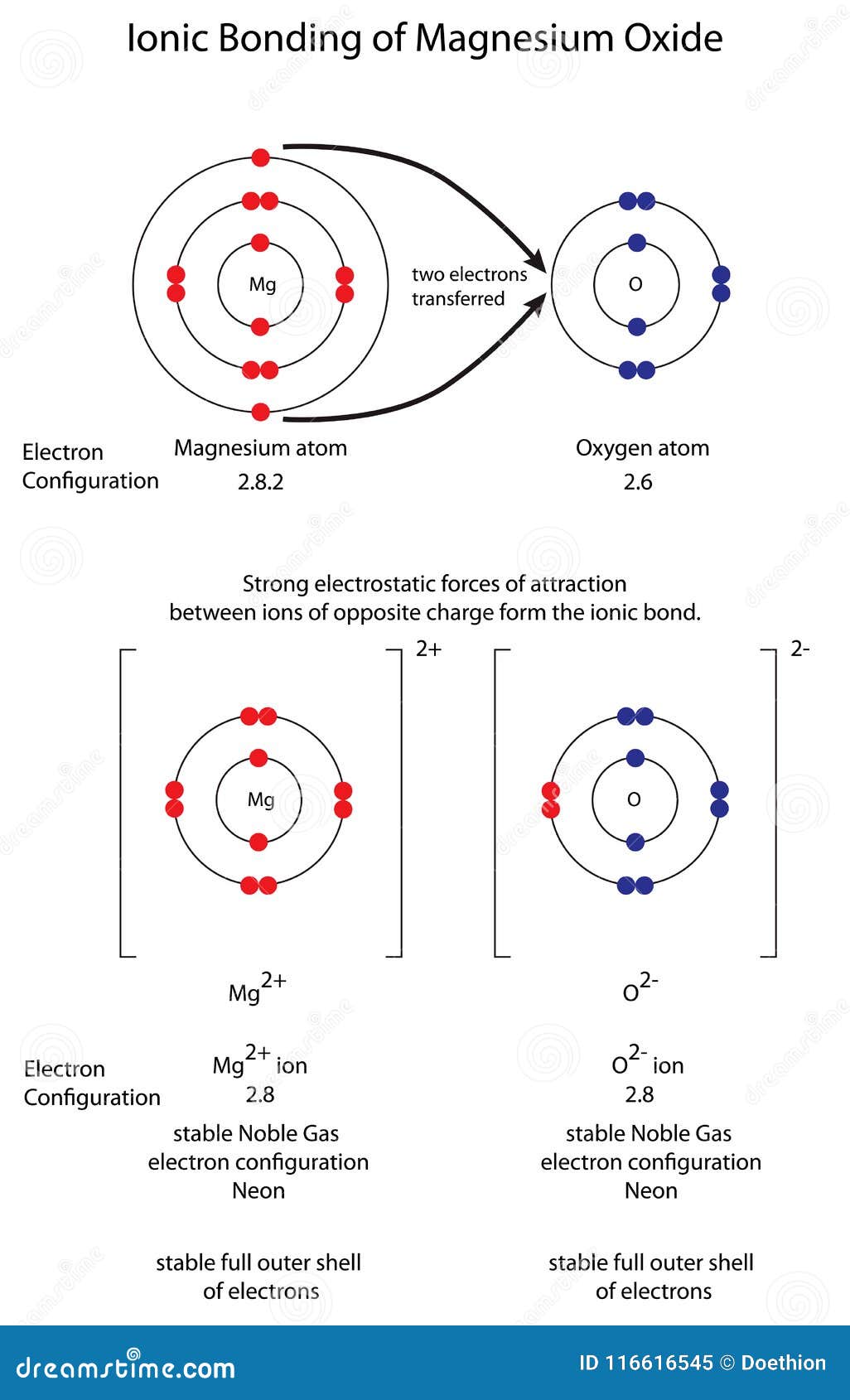
What Is An Ionic Bond Formed By . Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms. What is an ionic bond? Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. For both atoms involved, this exchange. These oppositely charged ions attract each other to form ionic networks (or lattices ). Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction.
from www.dreamstime.com
These oppositely charged ions attract each other to form ionic networks (or lattices ). Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms. For both atoms involved, this exchange. What is an ionic bond?
Diagram To Show Ionic Bonding in Magnesium Oxide MgO Stock Illustration
What Is An Ionic Bond Formed By Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms. What is an ionic bond? Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. For both atoms involved, this exchange. These oppositely charged ions attract each other to form ionic networks (or lattices ).
From logan-has-small.blogspot.com
What is an Ionic Bond LoganhasSmall What Is An Ionic Bond Formed By What is an ionic bond? An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. These oppositely charged ions attract each other to form ionic networks (or lattices ). Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ionic bonding. What Is An Ionic Bond Formed By.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk What Is An Ionic Bond Formed By For both atoms involved, this exchange. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions. What Is An Ionic Bond Formed By.
From tuitiontube.com
Ionic Bond and Ionic Bond Formation, Definition, Properties in What Is An Ionic Bond Formed By For both atoms involved, this exchange. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged. What Is An Ionic Bond Formed By.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii What Is An Ionic Bond Formed By Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from. What Is An Ionic Bond Formed By.
From www.sliderbase.com
Chemical Bonds What Is An Ionic Bond Formed By Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms. Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from. What Is An Ionic Bond Formed By.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? What Is An Ionic Bond Formed By Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: These oppositely charged ions attract each other to form ionic networks (or lattices ). Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. What is an ionic bond? For. What Is An Ionic Bond Formed By.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica What Is An Ionic Bond Formed By Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. Ionic. What Is An Ionic Bond Formed By.
From biologydictionary.net
Ionic Bond Examples Biology Dictionary What Is An Ionic Bond Formed By These oppositely charged ions attract each other to form ionic networks (or lattices ). Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. For both atoms involved, this exchange. Compounds composed. What Is An Ionic Bond Formed By.
From www.dreamstime.com
Diagram To Show Ionic Bonding in Magnesium Oxide MgO Stock Illustration What Is An Ionic Bond Formed By What is an ionic bond? Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. These oppositely charged ions attract each other to form ionic networks (or lattices ). Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic. What Is An Ionic Bond Formed By.
From www.chemistrylearner.com
Ionic Bond Facts, Definition, Properties, Examples, & Diagrams What Is An Ionic Bond Formed By For both atoms involved, this exchange. What is an ionic bond? Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. Ionic bond, type of linkage formed from the. What Is An Ionic Bond Formed By.
From www.slideserve.com
PPT IONIC BONDING PowerPoint Presentation, free download ID1785078 What Is An Ionic Bond Formed By These oppositely charged ions attract each other to form ionic networks (or lattices ). Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms. What is an ionic bond?. What Is An Ionic Bond Formed By.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures What Is An Ionic Bond Formed By Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. What is an ionic bond? An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged. What Is An Ionic Bond Formed By.
From pediaa.com
Difference Between Covalent and Ionic Bonds What Is An Ionic Bond Formed By What is an ionic bond? Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. For both atoms involved, this exchange. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bonding is a type of chemical bonding that involves the. What Is An Ionic Bond Formed By.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning What Is An Ionic Bond Formed By An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. What is an ionic bond? These oppositely charged ions attract each other to form ionic networks (or lattices ). Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:. What Is An Ionic Bond Formed By.
From www.youtube.com
Chemistry How Ionic Bonds (Electrovalent bonds) are formed Chemical What Is An Ionic Bond Formed By Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. What Is An Ionic Bond Formed By.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision What Is An Ionic Bond Formed By What is an ionic bond? Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held. What Is An Ionic Bond Formed By.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry What Is An Ionic Bond Formed By For both atoms involved, this exchange. These oppositely charged ions attract each other to form ionic networks (or lattices ). What is an ionic bond? An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions. What Is An Ionic Bond Formed By.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry What Is An Ionic Bond Formed By For both atoms involved, this exchange. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. What is an ionic bond? Ionic bonding is a type of chemical bonding. What Is An Ionic Bond Formed By.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures What Is An Ionic Bond Formed By Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms. Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. What Is An Ionic Bond Formed By.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts What Is An Ionic Bond Formed By Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: For both atoms involved, this exchange. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. These oppositely charged ions attract each other to form ionic networks (or lattices. What Is An Ionic Bond Formed By.
From www.chemistrylearner.com
Chemical Bonds Definition, Types, and Examples What Is An Ionic Bond Formed By What is an ionic bond? Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions. What Is An Ionic Bond Formed By.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica What Is An Ionic Bond Formed By Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. These oppositely charged ions attract each other to form ionic networks (or lattices ). Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bond, type of linkage formed. What Is An Ionic Bond Formed By.
From www.revisechemistry.uk
Bonding and Properties of materials OCR Gateway C2 revisechemistry.uk What Is An Ionic Bond Formed By Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms. Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. What Is An Ionic Bond Formed By.
From en.wikipedia.org
Ionic bonding Wikipedia What Is An Ionic Bond Formed By For both atoms involved, this exchange. What is an ionic bond? An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ionic bonding is a form of chemical connection in which. What Is An Ionic Bond Formed By.
From www.carlsonstockart.com
Ionic Bonding Carlson Stock Art What Is An Ionic Bond Formed By For both atoms involved, this exchange. These oppositely charged ions attract each other to form ionic networks (or lattices ). Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bonding. What Is An Ionic Bond Formed By.
From www.youtube.com
Ionic Bonding and Electron Configurations YouTube What Is An Ionic Bond Formed By For both atoms involved, this exchange. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: What is an ionic bond? Ionic bonding is a form of chemical connection in. What Is An Ionic Bond Formed By.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds What Is An Ionic Bond Formed By Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. For both. What Is An Ionic Bond Formed By.
From www2.victoriacollege.edu
formation of ionic bonds What Is An Ionic Bond Formed By Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic. What Is An Ionic Bond Formed By.
From www.youtube.com
How To Draw The Lewis Structures of Ionic Compounds YouTube What Is An Ionic Bond Formed By What is an ionic bond? An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged. What Is An Ionic Bond Formed By.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry What Is An Ionic Bond Formed By Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. These oppositely charged ions attract each other to form ionic networks (or lattices ). What is an ionic bond? Ionic. What Is An Ionic Bond Formed By.
From www.youtube.com
Examples of Ionic Bonding YouTube What Is An Ionic Bond Formed By Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. These. What Is An Ionic Bond Formed By.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds What Is An Ionic Bond Formed By These oppositely charged ions attract each other to form ionic networks (or lattices ). Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. What is an ionic bond? Ionic bonding is. What Is An Ionic Bond Formed By.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica What Is An Ionic Bond Formed By Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms. Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. These oppositely charged ions attract each other to form ionic networks (or lattices ). Ionic bond, type of linkage. What Is An Ionic Bond Formed By.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and What Is An Ionic Bond Formed By What is an ionic bond? An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction. For both atoms involved, this exchange. Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms. Ionic bond, type of linkage formed from. What Is An Ionic Bond Formed By.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5980343 What Is An Ionic Bond Formed By Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: What is an ionic bond? Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms. Ionic bonding is a form of chemical connection in which one atom loses valence. What Is An Ionic Bond Formed By.