Zinc Chloride Dissociation Equation . When nacl dissolves in water, the ions separate and go their own way in solution; The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. The ions are now written with their respective charges, and the (aq) phase label emphasizes that they are. 108k views 1 year ago. The chemical equation for this reaction is given by: The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. This is because of the \(2+\) charge of the calcium ion. Zn + 2hcl → zncl 2 + h 2 Solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride.
from www.chegg.com
Solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the. Zn + 2hcl → zncl 2 + h 2 When nacl dissolves in water, the ions separate and go their own way in solution; The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. The chemical equation for this reaction is given by: This is because of the \(2+\) charge of the calcium ion. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. 108k views 1 year ago. The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. The ions are now written with their respective charges, and the (aq) phase label emphasizes that they are.
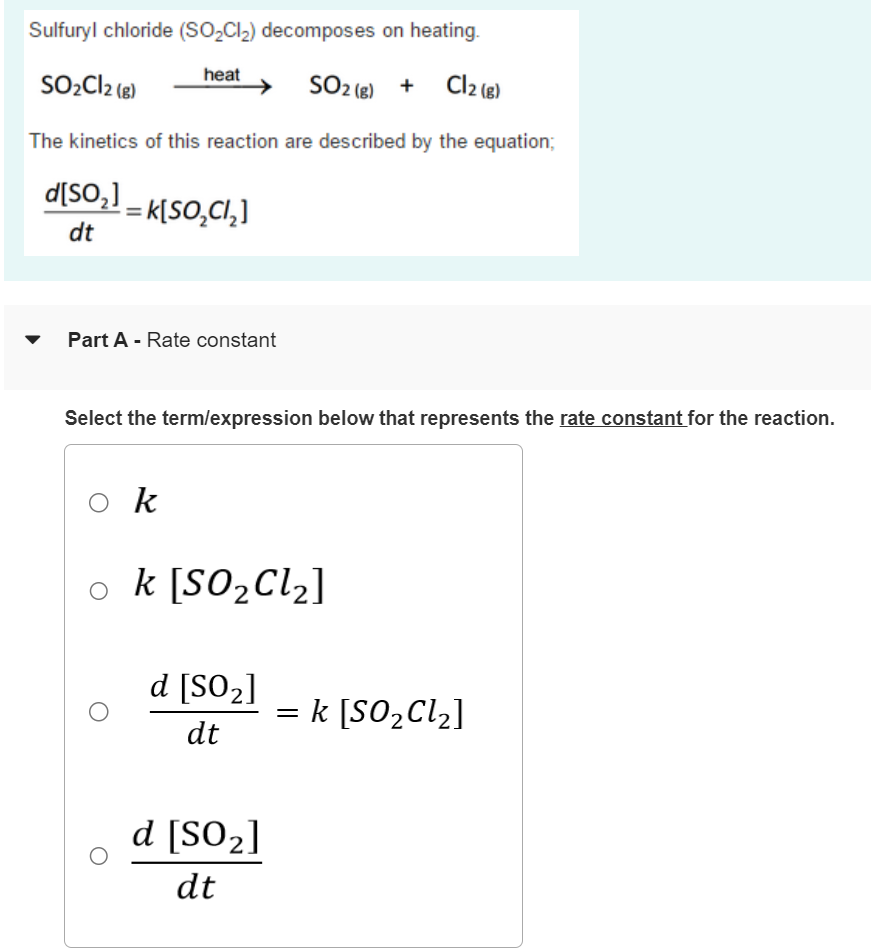
Solved Sulfuryl chloride (SO2Cl2) on heating.
Zinc Chloride Dissociation Equation The ions are now written with their respective charges, and the (aq) phase label emphasizes that they are. 108k views 1 year ago. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. The ions are now written with their respective charges, and the (aq) phase label emphasizes that they are. Solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the. This is because of the \(2+\) charge of the calcium ion. The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. Zn + 2hcl → zncl 2 + h 2 The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. The chemical equation for this reaction is given by: When nacl dissolves in water, the ions separate and go their own way in solution;
From www.slideserve.com
PPT Isotonic Solutions PowerPoint Presentation, free download ID Zinc Chloride Dissociation Equation The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. This is because of the \(2+\) charge of the calcium ion. The chemical equation for. Zinc Chloride Dissociation Equation.
From www.chemicals.co.uk
Practical GCSE Chemistry Making Salts The Science Blog Zinc Chloride Dissociation Equation When nacl dissolves in water, the ions separate and go their own way in solution; The chemical equation for this reaction is given by: 108k views 1 year ago. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. This is because of the \(2+\) charge of the calcium ion. The ions are now. Zinc Chloride Dissociation Equation.
From www.numerade.com
SOLVED A 5 gram sample of zinc is added to hydrochloric acid. The Zinc Chloride Dissociation Equation Solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. This is because of the \(2+\) charge of the calcium. Zinc Chloride Dissociation Equation.
From www.tessshebaylo.com
Salt Water Electrolysis Equation Tessshebaylo Zinc Chloride Dissociation Equation Zn + 2hcl → zncl 2 + h 2 The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. This is because of the \(2+\) charge of the calcium ion. 108k views 1 year ago. When nacl dissolves in. Zinc Chloride Dissociation Equation.
From www.chegg.com
Solved 2. Iron (III) chloride dissolves in water forming Zinc Chloride Dissociation Equation The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. When nacl dissolves in water, the ions separate and go their own way in solution; The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride.. Zinc Chloride Dissociation Equation.
From www.doubtnut.com
In passing chlorine gas through a concentrated solution of alkali we g Zinc Chloride Dissociation Equation 108k views 1 year ago. When nacl dissolves in water, the ions separate and go their own way in solution; The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. The chemical equation for this reaction is given by: The ions are now written with their respective charges, and the (aq) phase label emphasizes. Zinc Chloride Dissociation Equation.
From www.toppr.com
5. Write a balanced chemical equations the following chemical reactions Zinc Chloride Dissociation Equation This is because of the \(2+\) charge of the calcium ion. The ions are now written with their respective charges, and the (aq) phase label emphasizes that they are. When nacl dissolves in water, the ions separate and go their own way in solution; The chemical equation for this reaction is given by: 108k views 1 year ago. The calcium. Zinc Chloride Dissociation Equation.
From express.adobe.com
Zinc and Copper Chloride Zinc Chloride Dissociation Equation 108k views 1 year ago. When nacl dissolves in water, the ions separate and go their own way in solution; The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. Zn + 2hcl → zncl 2 + h 2 This is. Zinc Chloride Dissociation Equation.
From shotprofessional22.gitlab.io
Divine Zinc Plus Hydrochloric Acid Balanced Equation Different Formulas Zinc Chloride Dissociation Equation The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. When nacl dissolves in water, the ions separate and go their own way in solution; The chemical equation for this reaction is given by: 108k views 1 year ago. This is because of the \(2+\) charge of the calcium ion. The reaction between metallic zinc. Zinc Chloride Dissociation Equation.
From www.chegg.com
Solved Lead (IV) oxide dissolves in concentrated Zinc Chloride Dissociation Equation The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. The ions are now written with their respective charges, and the (aq) phase label emphasizes that they are. This is because of the \(2+\) charge of the calcium ion. Zn + 2hcl → zncl 2 + h 2 108k views 1 year ago. The reaction between. Zinc Chloride Dissociation Equation.
From www.scielo.br
SciELO Brasil Synthesis and Characterization of Zinc Oxide Obtained Zinc Chloride Dissociation Equation Zn + 2hcl → zncl 2 + h 2 Solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the. The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. 108k views 1 year ago. This is because of the \(2+\) charge of the calcium ion.. Zinc Chloride Dissociation Equation.
From www.chegg.com
Solved Sulfuryl chloride (SO2Cl2) on heating. Zinc Chloride Dissociation Equation The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. When nacl dissolves in water, the ions separate and go their own way in solution; The chemical equation for this reaction is given by: 108k views 1 year ago. The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. This. Zinc Chloride Dissociation Equation.
From asideload7.gitlab.io
Ideal Balanced Chemical Equation Hcl And Naoh Equations Worksheet With Zinc Chloride Dissociation Equation Zn + 2hcl → zncl 2 + h 2 The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. This is because of the \(2+\) charge of the calcium ion. The ions are now written with their respective charges, and the (aq) phase label emphasizes that they are. The chemical equation for this reaction. Zinc Chloride Dissociation Equation.
From www.numerade.com
SOLVED The equation for dissolving sodium chloride in water is given Zinc Chloride Dissociation Equation 108k views 1 year ago. The ions are now written with their respective charges, and the (aq) phase label emphasizes that they are. When nacl dissolves in water, the ions separate and go their own way in solution; Solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the. The calcium. Zinc Chloride Dissociation Equation.
From www.youtube.com
How to Write the Formula for Zinc chloride (ZnCl2) YouTube Zinc Chloride Dissociation Equation The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. This is because of the \(2+\) charge of the calcium ion. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. Solubility equilibrium defines the. Zinc Chloride Dissociation Equation.
From www.slideserve.com
PPT Isotonic Solutions PowerPoint Presentation, free download ID Zinc Chloride Dissociation Equation The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. Solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the. The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. The chemical equation for this reaction is given by: When nacl. Zinc Chloride Dissociation Equation.
From www.youtube.com
Equation for CrCl3 + H2O Chromium (III) chloride + Water YouTube Zinc Chloride Dissociation Equation The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. The ions are now written with their respective charges, and the (aq) phase label emphasizes that they are. This is because of the \(2+\) charge of the calcium ion. When nacl dissolves in water, the ions separate and go their own way in solution;. Zinc Chloride Dissociation Equation.
From www.numerade.com
SOLVED A chloride dissolves in water to form a colored solution. The Zinc Chloride Dissociation Equation This is because of the \(2+\) charge of the calcium ion. The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. 108k views 1 year ago. Zn + 2hcl → zncl 2 + h 2 The reaction between metallic zinc and. Zinc Chloride Dissociation Equation.
From www.youtube.com
How to Write the Net Ionic Equation for ZnCl2 + NaOH = Zn(OH)2 + NaCl Zinc Chloride Dissociation Equation Solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the. This is because of the \(2+\) charge of the calcium ion. The chemical equation for this reaction is given by: The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. The ions are now written. Zinc Chloride Dissociation Equation.
From www.numerade.com
SOLVED Question 14 (5 points) Calculate how many moles of zinc Zinc Chloride Dissociation Equation When nacl dissolves in water, the ions separate and go their own way in solution; The ions are now written with their respective charges, and the (aq) phase label emphasizes that they are. The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. The chemical equation for this reaction is given by: This is because of. Zinc Chloride Dissociation Equation.
From www.chegg.com
Solved The compound cobalt(II) chloride, CoCl2 is soluble in Zinc Chloride Dissociation Equation Zn + 2hcl → zncl 2 + h 2 The ions are now written with their respective charges, and the (aq) phase label emphasizes that they are. This is because of the \(2+\) charge of the calcium ion. 108k views 1 year ago. The chemical equation for this reaction is given by: The reaction between metallic zinc and hydrogen chloride. Zinc Chloride Dissociation Equation.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Zinc Chloride Dissociation Equation When nacl dissolves in water, the ions separate and go their own way in solution; The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. 108k views 1 year ago. Zn + 2hcl → zncl 2 + h 2 The reaction. Zinc Chloride Dissociation Equation.
From www.pakistanchemical.com
Zinc Chloride PAKISTAN CHEMICAL Zinc Chloride Dissociation Equation The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. The chemical equation for this reaction is given by: Zn + 2hcl → zncl 2 + h 2 108k views 1 year ago. When nacl dissolves in water, the ions separate and go their own way in solution; The reaction between metallic zinc and hydrogen. Zinc Chloride Dissociation Equation.
From www.numerade.com
SOLVED Write dissociation reactions of the following ionic compounds Zinc Chloride Dissociation Equation When nacl dissolves in water, the ions separate and go their own way in solution; The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. 108k views 1 year ago. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. The calcium nitrate formula unit dissociates into one calcium. Zinc Chloride Dissociation Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + Cu(NO3)2 = Cu + Zn(NO3)2 Zinc Chloride Dissociation Equation 108k views 1 year ago. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. The ions are now written with their respective charges, and the (aq) phase label emphasizes that they are. This is because of the \(2+\) charge of the calcium ion. The chemical equation for this reaction is given by: Solubility. Zinc Chloride Dissociation Equation.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Zinc Chloride Dissociation Equation The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. Zn + 2hcl → zncl 2 + h 2 The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. This is because of the \(2+\) charge of the calcium ion. Solubility equilibrium defines the dynamic equilibria between a precipitate and its. Zinc Chloride Dissociation Equation.
From www.bartleby.com
Answered The compound zinc chloride, ZnCl, is… bartleby Zinc Chloride Dissociation Equation The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. When nacl dissolves in water, the ions separate and go their own way in solution; The ions are now written with their respective charges, and the (aq) phase label emphasizes that they are. The formula unit of sodium chloride dissociates into one sodium ion. Zinc Chloride Dissociation Equation.
From www.numerade.com
SOLVED REACTION5COPPER SULFATE(CuSO,)AND SODIUM HYDROXIDE(NaOH) (Write Zinc Chloride Dissociation Equation Solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the. The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. The ions are now written with their respective charges, and the (aq) phase label emphasizes that they are. The reaction between metallic zinc and hydrogen chloride. Zinc Chloride Dissociation Equation.
From www.researchgate.net
(PDF) Review of Ammonium ChlorideWater Solution Properties Zinc Chloride Dissociation Equation Zn + 2hcl → zncl 2 + h 2 The chemical equation for this reaction is given by: This is because of the \(2+\) charge of the calcium ion. The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. The ions are now written with their respective charges, and the (aq) phase label emphasizes that they. Zinc Chloride Dissociation Equation.
From www.youtube.com
How to Balance NaCl = Na + Cl2 (Electrolysis of Sodium chloride ) YouTube Zinc Chloride Dissociation Equation When nacl dissolves in water, the ions separate and go their own way in solution; This is because of the \(2+\) charge of the calcium ion. Zn + 2hcl → zncl 2 + h 2 The chemical equation for this reaction is given by: 108k views 1 year ago. The formula unit of sodium chloride dissociates into one sodium ion. Zinc Chloride Dissociation Equation.
From hxelxlglm.blob.core.windows.net
Calcium Phosphate Dissociation Equation at Donald Floyd blog Zinc Chloride Dissociation Equation Zn + 2hcl → zncl 2 + h 2 Solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the. The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride.. Zinc Chloride Dissociation Equation.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc Chloride Dissociation Equation 108k views 1 year ago. The ions are now written with their respective charges, and the (aq) phase label emphasizes that they are. The chemical equation for this reaction is given by: This is because of the \(2+\) charge of the calcium ion. The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. The calcium. Zinc Chloride Dissociation Equation.
From www.youtube.com
Equation for ZnCl2 + H2O (Zinc chloride + Water) YouTube Zinc Chloride Dissociation Equation 108k views 1 year ago. Solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. This is because of the. Zinc Chloride Dissociation Equation.
From www.chegg.com
Solved REPORT SHEET Chemical Formulas A, Zinc Chloride 1. Zinc Chloride Dissociation Equation When nacl dissolves in water, the ions separate and go their own way in solution; Solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals the. This is because of the \(2+\) charge of the calcium ion. 108k views 1 year ago. The formula unit of sodium chloride dissociates into one. Zinc Chloride Dissociation Equation.
From byjus.com
Zinc and hydrochloric acid react according t reaction Zn(s) + 2HCI(aq Zinc Chloride Dissociation Equation The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. This is because of the \(2+\) charge of the calcium ion. When nacl dissolves in water, the ions separate and go their own way in solution; Solubility equilibrium defines the dynamic equilibria between a precipitate and its dissolved ions when the rate of dissolution equals. Zinc Chloride Dissociation Equation.