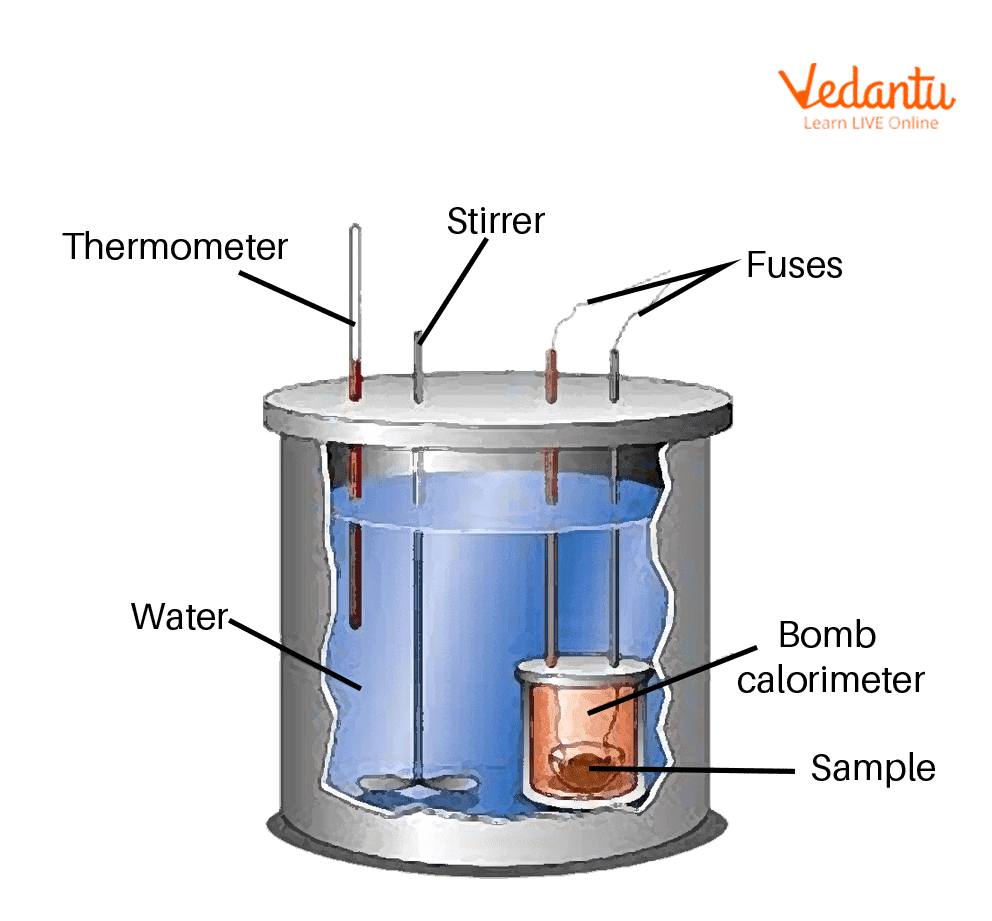
Bomb Calorimeter Ap Chemistry . Compare and contrast coffee cup calorimetry and bomb calorimetry. What is a bomb calorimeter? 6000a bomb calorimeter has a heat capacity of 3.18 kj/k. The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. A typical bomb calorimetry set up is shown here. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. Revision notes on calorimetry calculations for the college board ap chemistry syllabus, written by the chemistry experts at. A different type of calorimeter that operates at constant volume, colloquially known as a bomb calorimeter, is used to measure the energy. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a wide variety of reactions. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calculate the amount of heat needed. The modern bomb calorimeter is a development of the original calorimeter of Aluminum metal can be recycled from scrap metal by melting the metal to evaporate.
from www.vedantu.com
The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. What is a bomb calorimeter? A different type of calorimeter that operates at constant volume, colloquially known as a bomb calorimeter, is used to measure the energy. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. The modern bomb calorimeter is a development of the original calorimeter of Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a wide variety of reactions. Compare and contrast coffee cup calorimetry and bomb calorimetry. Revision notes on calorimetry calculations for the college board ap chemistry syllabus, written by the chemistry experts at.
Bomb Calorimeter Learn Important Terms and Concepts
Bomb Calorimeter Ap Chemistry 6000a bomb calorimeter has a heat capacity of 3.18 kj/k. The modern bomb calorimeter is a development of the original calorimeter of A different type of calorimeter that operates at constant volume, colloquially known as a bomb calorimeter, is used to measure the energy. Compare and contrast coffee cup calorimetry and bomb calorimetry. The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. What is a bomb calorimeter? 6000a bomb calorimeter has a heat capacity of 3.18 kj/k. Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Revision notes on calorimetry calculations for the college board ap chemistry syllabus, written by the chemistry experts at. Calculate the amount of heat needed. A typical bomb calorimetry set up is shown here. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a wide variety of reactions.
From www.thoughtco.com
Calorimeter Definition in Chemistry Bomb Calorimeter Ap Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A different type of calorimeter that operates at constant volume, colloquially known as a bomb calorimeter, is used to measure the energy. The modern bomb calorimeter is a development of the original calorimeter of Compare and contrast coffee cup calorimetry and bomb. Bomb Calorimeter Ap Chemistry.
From www.youtube.com
Bomb calorimeter construction of bomb calorimeter working of bomb Bomb Calorimeter Ap Chemistry 6000a bomb calorimeter has a heat capacity of 3.18 kj/k. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. A typical bomb calorimetry set up is shown here. The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. What is a bomb. Bomb Calorimeter Ap Chemistry.
From chemistrytalk.org
Calorimetry ChemTalk Bomb Calorimeter Ap Chemistry Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a wide variety of reactions. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. Compare and contrast coffee. Bomb Calorimeter Ap Chemistry.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation Bomb Calorimeter Ap Chemistry The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Compare and contrast coffee cup calorimetry and bomb calorimetry. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. 6000a bomb calorimeter has a heat capacity of 3.18 kj/k. Calculate heat, temperature. Bomb Calorimeter Ap Chemistry.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Bomb Calorimeter Ap Chemistry Revision notes on calorimetry calculations for the college board ap chemistry syllabus, written by the chemistry experts at. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a wide variety of reactions. A. Bomb Calorimeter Ap Chemistry.
From www.slideserve.com
PPT Chapter 6 Thermochemistry PowerPoint Presentation, free download Bomb Calorimeter Ap Chemistry Calculate the amount of heat needed. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. The modern bomb calorimeter is a development of the original calorimeter of Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A typical bomb calorimetry set up is shown here. Bomb calorimetry. Bomb Calorimeter Ap Chemistry.
From byjus.com
What is bomb calorimeter? Bomb Calorimeter Ap Chemistry The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. Aluminum metal can be recycled from scrap metal. Bomb Calorimeter Ap Chemistry.
From www.labster.com
Calorimetry Using a bomb calorimeter (NEW) Labster Bomb Calorimeter Ap Chemistry Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a wide variety of reactions. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calculate heat, temperature change,. Bomb Calorimeter Ap Chemistry.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimeter Ap Chemistry Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of. Bomb Calorimeter Ap Chemistry.
From www.scribd.com
Bomb Calorimeter Principle,formula procedure.docx Calorimetry Enthalpy Bomb Calorimeter Ap Chemistry Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. The modern bomb calorimeter is a development of the original calorimeter of Revision notes on calorimetry calculations for the college board ap chemistry syllabus, written by the. Bomb Calorimeter Ap Chemistry.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Bomb Calorimeter Ap Chemistry The modern bomb calorimeter is a development of the original calorimeter of A different type of calorimeter that operates at constant volume, colloquially known as a bomb calorimeter, is used to measure the energy. What is a bomb calorimeter? Compare and contrast coffee cup calorimetry and bomb calorimetry. 6000a bomb calorimeter has a heat capacity of 3.18 kj/k. Aluminum metal. Bomb Calorimeter Ap Chemistry.
From studylib.net
1b bomb calorimeter Bomb Calorimeter Ap Chemistry Revision notes on calorimetry calculations for the college board ap chemistry syllabus, written by the chemistry experts at. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. A typical bomb calorimetry set up is shown here. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume.. Bomb Calorimeter Ap Chemistry.
From ddscalorimeters.com
CAL3KAP Calorimeter Bomb Calorimeter Ap Chemistry Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. Calculate the amount of heat needed. The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. Compare and contrast coffee cup calorimetry and bomb calorimetry. Aluminum metal can be recycled from. Bomb Calorimeter Ap Chemistry.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimeter Ap Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. Constant volume calorimetry, also know as bomb calorimetry, is. Bomb Calorimeter Ap Chemistry.
From www.shutterstock.com
Bomb Calorimeter Vector Illustration Labeled Educational Stock Vector Bomb Calorimeter Ap Chemistry Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. Bomb Calorimeter Ap Chemistry.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Ap Chemistry 6000a bomb calorimeter has a heat capacity of 3.18 kj/k. The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. Calculate the amount of heat needed. Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. A different type of calorimeter that operates at constant volume, colloquially. Bomb Calorimeter Ap Chemistry.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube Bomb Calorimeter Ap Chemistry Revision notes on calorimetry calculations for the college board ap chemistry syllabus, written by the chemistry experts at. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. A different type of calorimeter that operates at constant. Bomb Calorimeter Ap Chemistry.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimeter Ap Chemistry Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calculate the amount of heat needed. 6000a bomb calorimeter has a heat capacity of 3.18 kj/k. Revision notes on calorimetry calculations for the college board ap chemistry syllabus, written by the chemistry experts at. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between. Bomb Calorimeter Ap Chemistry.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Ap Chemistry A different type of calorimeter that operates at constant volume, colloquially known as a bomb calorimeter, is used to measure the energy. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Compare and contrast coffee cup calorimetry and bomb calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is. Bomb Calorimeter Ap Chemistry.
From slideplayer.com
AP Chemistry Unit 5 Thermochemistry. ppt download Bomb Calorimeter Ap Chemistry 6000a bomb calorimeter has a heat capacity of 3.18 kj/k. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. Revision notes on calorimetry calculations for the college board ap chemistry syllabus, written by the chemistry experts at. Compare and contrast coffee cup calorimetry and bomb calorimetry. Calculate the amount. Bomb Calorimeter Ap Chemistry.
From www.researchgate.net
Schematic sketch of a bomb calorimeter Download Scientific Diagram Bomb Calorimeter Ap Chemistry What is a bomb calorimeter? Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. Revision notes on calorimetry calculations for the college board ap chemistry syllabus, written by the chemistry experts at. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. Bomb Calorimeter Ap Chemistry.
From shaunmwilliams.com
Chapter 6 Presentation Bomb Calorimeter Ap Chemistry A different type of calorimeter that operates at constant volume, colloquially known as a bomb calorimeter, is used to measure the energy. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. A typical bomb calorimetry set up is shown here. The reaction is contained in a heavy metallic container. Bomb Calorimeter Ap Chemistry.
From wingle.jp
😂 Bomb calorimeter experiment conclusion. Experiment 1 A3 Bomb Bomb Calorimeter Ap Chemistry The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. What is a bomb calorimeter? Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. A typical bomb calorimetry set up is shown here. Revision notes on calorimetry calculations for the college board ap chemistry syllabus, written by. Bomb Calorimeter Ap Chemistry.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Bomb Calorimeter Ap Chemistry Bomb calorimetry is used predominantly to measure the heat evolved in combustion reactions, but can be used for a wide variety of reactions. Calculate the amount of heat needed. The modern bomb calorimeter is a development of the original calorimeter of Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. The reaction is contained in a. Bomb Calorimeter Ap Chemistry.
From www.slideserve.com
PPT Chemistry A Molecular Approach , 1 st Edition Nivaldo Tro Bomb Calorimeter Ap Chemistry The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. Calculate the amount of heat needed. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. Bomb Calorimeter Ap Chemistry.
From socratic.org
When 0.602 g of biphenyl (C12H10) undergoes combustion in a bomb Bomb Calorimeter Ap Chemistry Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Compare and contrast coffee cup calorimetry and bomb calorimetry. Revision notes on calorimetry calculations for the college board ap chemistry syllabus, written by the chemistry experts at. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume.. Bomb Calorimeter Ap Chemistry.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimeter Ap Chemistry 6000a bomb calorimeter has a heat capacity of 3.18 kj/k. A typical bomb calorimetry set up is shown here. Revision notes on calorimetry calculations for the college board ap chemistry syllabus, written by the chemistry experts at. Compare and contrast coffee cup calorimetry and bomb calorimetry. A different type of calorimeter that operates at constant volume, colloquially known as a. Bomb Calorimeter Ap Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry I Bomb Calorimeter Ap Chemistry The modern bomb calorimeter is a development of the original calorimeter of Compare and contrast coffee cup calorimetry and bomb calorimetry. The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Constant volume. Bomb Calorimeter Ap Chemistry.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Bomb Calorimeter Ap Chemistry Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. What is a bomb calorimeter? Calculate the amount of heat needed. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Bomb calorimetry is used. Bomb Calorimeter Ap Chemistry.
From saylordotorg.github.io
Calorimetry Bomb Calorimeter Ap Chemistry What is a bomb calorimeter? 6000a bomb calorimeter has a heat capacity of 3.18 kj/k. Aluminum metal can be recycled from scrap metal by melting the metal to evaporate. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. Calculate the molar heat of enthalpy for a reactions using coffee. Bomb Calorimeter Ap Chemistry.
From studylib.net
Bomb Calorimeter Bomb Calorimeter Ap Chemistry Calculate the amount of heat needed. What is a bomb calorimeter? The modern bomb calorimeter is a development of the original calorimeter of Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. Bomb calorimetry is used. Bomb Calorimeter Ap Chemistry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID9276632 Bomb Calorimeter Ap Chemistry A different type of calorimeter that operates at constant volume, colloquially known as a bomb calorimeter, is used to measure the energy. What is a bomb calorimeter? Compare and contrast coffee cup calorimetry and bomb calorimetry. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Calculate the amount of heat needed.. Bomb Calorimeter Ap Chemistry.
From scitechdidactic.com
Bomb Calorimeter Model TH 101 Scitech Didactic UK Bomb Calorimeter Ap Chemistry The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. The modern bomb calorimeter is a development of the original calorimeter of The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at. Bomb Calorimeter Ap Chemistry.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Bomb Calorimeter Ap Chemistry Calculate the amount of heat needed. A typical bomb calorimetry set up is shown here. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. The calorimeter used to determine the energy change during a reaction accurately. Bomb Calorimeter Ap Chemistry.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Bomb Calorimeter Ap Chemistry 6000a bomb calorimeter has a heat capacity of 3.18 kj/k. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. The reaction is contained in a heavy metallic container (the bomb) forcing the reaction to occur at constant volume. Compare and contrast coffee cup calorimetry and bomb calorimetry. Calculate the amount of heat needed. A different type. Bomb Calorimeter Ap Chemistry.