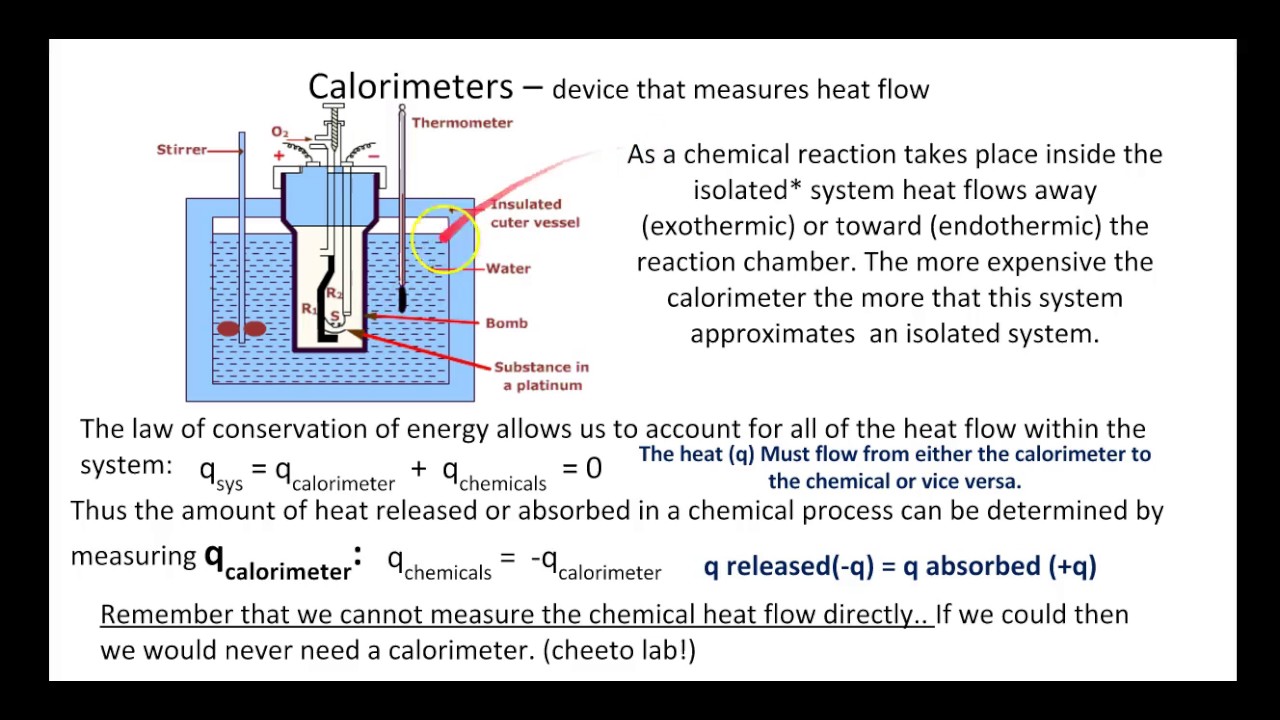
Calorimetry Examples In Chemistry . Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Suppose we initially have a high. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the When 50.0 g of 0.200 m nacl (aq) at 24.1 °c is added to 100.0 g of 0.100 m agno 3 (aq) at 24.1 °c in a calorimeter, the temperature. Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen.
from jiahui-fahrenheitnanime.blogspot.com
Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Suppose we initially have a high. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. When 50.0 g of 0.200 m nacl (aq) at 24.1 °c is added to 100.0 g of 0.100 m agno 3 (aq) at 24.1 °c in a calorimeter, the temperature. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the
Calorimetry Worksheet
Calorimetry Examples In Chemistry When 50.0 g of 0.200 m nacl (aq) at 24.1 °c is added to 100.0 g of 0.100 m agno 3 (aq) at 24.1 °c in a calorimeter, the temperature. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. When 50.0 g of 0.200 m nacl (aq) at 24.1 °c is added to 100.0 g of 0.100 m agno 3 (aq) at 24.1 °c in a calorimeter, the temperature. In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Suppose we initially have a high.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimetry Examples In Chemistry Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between. Calorimetry Examples In Chemistry.
From www.showme.com
4 steps for solving calorimetry problems Science, Chemistry ShowMe Calorimetry Examples In Chemistry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Suppose we initially have a high. Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. The amount of. Calorimetry Examples In Chemistry.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Examples In Chemistry Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates. Calorimetry Examples In Chemistry.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calorimetry Examples In Chemistry The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the When 50.0 g of 0.200 m nacl (aq) at 24.1 °c is added to 100.0 g of 0.100 m agno 3 (aq) at 24.1 °c in a calorimeter, the temperature. Suppose we initially have a high. Calculate heat, temperature change,. Calorimetry Examples In Chemistry.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube Calorimetry Examples In Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry.. Calorimetry Examples In Chemistry.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimetry Examples In Chemistry Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Suppose we initially have a high. Calculate the. Calorimetry Examples In Chemistry.
From jiahui-fahrenheitnanime.blogspot.com
Calorimetry Worksheet Calorimetry Examples In Chemistry Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the Before we practice calorimetry problems involving chemical reactions, consider. Calorimetry Examples In Chemistry.
From saylordotorg.github.io
Calorimetry Calorimetry Examples In Chemistry Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the When 50.0 g of 0.200. Calorimetry Examples In Chemistry.
From www.scribd.com
calorimetry Calorimetry Examples In Chemistry In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Compare heat flow from hot to cold objects in an ideal calorimeter versus a. Calorimetry Examples In Chemistry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Examples In Chemistry When 50.0 g of 0.200 m nacl (aq) at 24.1 °c is added to 100.0 g of 0.100 m agno 3 (aq) at 24.1 °c in a calorimeter, the temperature. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the Before discussing the calorimetry of chemical reactions, consider a simpler. Calorimetry Examples In Chemistry.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube Calorimetry Examples In Chemistry When 50.0 g of 0.200 m nacl (aq) at 24.1 °c is added to 100.0 g of 0.100 m agno 3 (aq) at 24.1 °c in a calorimeter, the temperature. Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Before we practice calorimetry problems involving chemical reactions, consider a simpler example. Calorimetry Examples In Chemistry.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Examples In Chemistry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Suppose we initially have a high. Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Calorimetry. Calorimetry Examples In Chemistry.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimetry Examples In Chemistry The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calculate heat, temperature change, and specific. Calorimetry Examples In Chemistry.
From www.studocu.com
Calorimetry Examples in Chemistry Chapter 9 Studocu Calorimetry Examples In Chemistry Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. When 50.0 g of 0.200 m nacl (aq) at 24.1 °c is added to 100.0 g of 0.100 m agno 3 (aq) at 24.1 °c in a calorimeter, the temperature. The amount of heat released or absorbed during a reaction is. Calorimetry Examples In Chemistry.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Examples In Chemistry Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Suppose we initially have a high. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The amount. Calorimetry Examples In Chemistry.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample Calorimetry Examples In Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Suppose we initially have a high. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. In a. Calorimetry Examples In Chemistry.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Examples In Chemistry Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the Before we practice calorimetry problems involving chemical reactions, consider. Calorimetry Examples In Chemistry.
From www.slideserve.com
PPT Q = MCΔT Specific Heat & CALORIMETRY PowerPoint Presentation Calorimetry Examples In Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Compare heat flow from hot to cold objects in an. Calorimetry Examples In Chemistry.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3207088 Calorimetry Examples In Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. The amount of heat released or absorbed during a reaction. Calorimetry Examples In Chemistry.
From www.youtube.com
General Chemistry II Solving Calorimetry Problems Neutralization Calorimetry Examples In Chemistry The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Before discussing the calorimetry of. Calorimetry Examples In Chemistry.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimetry Examples In Chemistry Suppose we initially have a high. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. When 50.0 g. Calorimetry Examples In Chemistry.
From studylib.net
Calorimetry Calorimetry Examples In Chemistry Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Suppose we initially have a high. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Before we practice calorimetry problems involving. Calorimetry Examples In Chemistry.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample Calorimetry Examples In Chemistry Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the When 50.0 g of 0.200 m nacl (aq) at 24.1 °c is added to 100.0 g of 0.100 m agno 3 (aq) at. Calorimetry Examples In Chemistry.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry Examples In Chemistry When 50.0 g of 0.200 m nacl (aq) at 24.1 °c is added to 100.0 g of 0.100 m agno 3 (aq) at 24.1 °c in a calorimeter, the temperature. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature. Calorimetry Examples In Chemistry.
From chemistrytalk.org
Calorimetry ChemTalk Calorimetry Examples In Chemistry Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Before we practice calorimetry problems involving chemical reactions, consider a simpler example. Calorimetry Examples In Chemistry.
From www.youtube.com
Unit 6 Calorimetry YouTube Calorimetry Examples In Chemistry Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Compare heat flow from hot to cold objects in an. Calorimetry Examples In Chemistry.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimetry Examples In Chemistry Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Suppose we initially have a high. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. When 50.0 g of 0.200 m nacl (aq). Calorimetry Examples In Chemistry.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetry Examples In Chemistry Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Suppose we initially have a high. Calorimetry. Calorimetry Examples In Chemistry.
From www.wizeprep.com
Calorimetry Wize University Chemistry Textbook Wizeprep Calorimetry Examples In Chemistry Suppose we initially have a high. Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.. Calorimetry Examples In Chemistry.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Calorimetry Examples In Chemistry The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Before discussing the calorimetry of chemical reactions, consider a. Calorimetry Examples In Chemistry.
From users.highland.edu
Calorimetry Calorimetry Examples In Chemistry Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Suppose we initially have a high. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the In a calorimetry experiment,. Calorimetry Examples In Chemistry.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Examples In Chemistry In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Suppose we initially have a high. When 50.0 g of 0.200 m nacl (aq). Calorimetry Examples In Chemistry.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample Calorimetry Examples In Chemistry Suppose we initially have a high. In a calorimetry experiment, 2.50 g of methane is burnt in excess oxygen. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The amount of heat released or absorbed during a reaction. Calorimetry Examples In Chemistry.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Examples In Chemistry Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the. Calorimetry Examples In Chemistry.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Examples In Chemistry Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Suppose we initially have a high. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. In a calorimetry experiment, 2.50 g of. Calorimetry Examples In Chemistry.