Electrolytes And Electrodes Functions . Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Electrodes and electrolytes are integral components of electrochemical systems, each possessing unique attributes that contribute to. Electrolytes are minerals with positive or negative charges like sodium, calcium, and potassium. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions) across themselves and to other cells. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood.
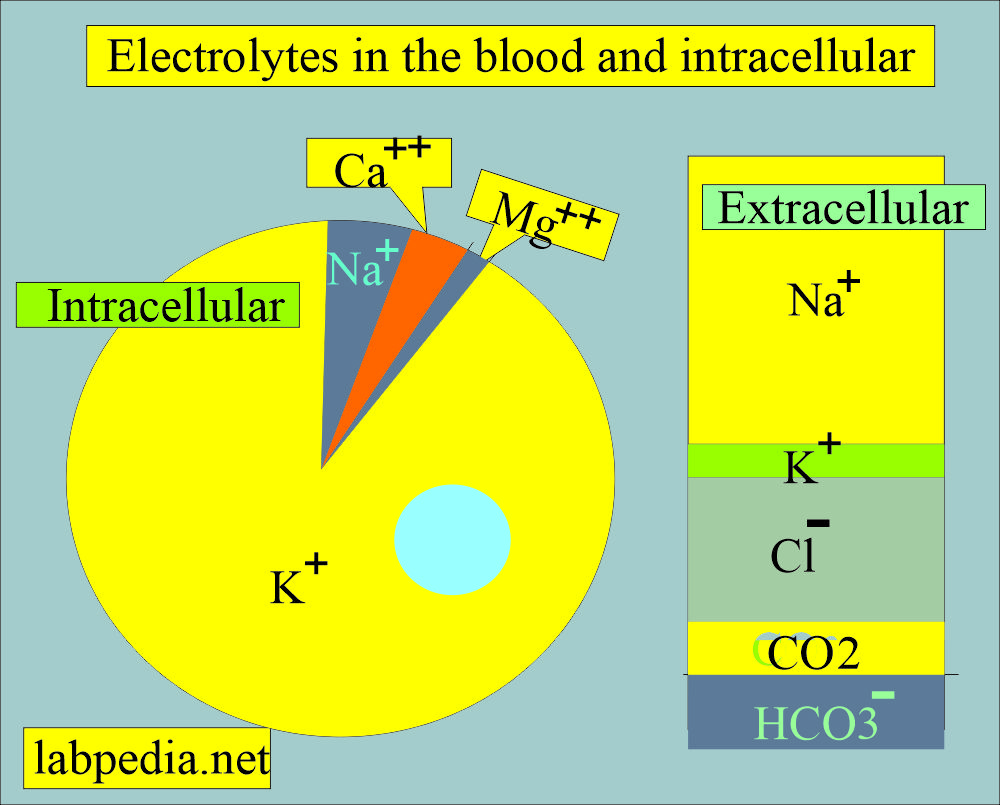
from labpedia.net
Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions) across themselves and to other cells. Electrodes and electrolytes are integral components of electrochemical systems, each possessing unique attributes that contribute to. Electrolytes are minerals with positive or negative charges like sodium, calcium, and potassium. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Your body needs electrolytes for brain function, muscle contractions, and managing blood ph.
Electrolytes Part 3 Electrolytes Panel
Electrolytes And Electrodes Functions Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions) across themselves and to other cells. Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Electrolytes are minerals with positive or negative charges like sodium, calcium, and potassium. Electrodes and electrolytes are integral components of electrochemical systems, each possessing unique attributes that contribute to. Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Electrolytes And Electrodes Functions Electrodes and electrolytes are integral components of electrochemical systems, each possessing unique attributes that contribute to. Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle. Electrolytes And Electrodes Functions.
From labpedia.net
Electrolytes Part 3 Electrolytes Panel Electrolytes And Electrodes Functions Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Electrodes and electrolytes are integral components of electrochemical systems, each possessing unique attributes that contribute to. Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Your body needs electrolytes for brain function, muscle contractions, and managing blood. Electrolytes And Electrodes Functions.
From www.phartoonz.com
List of electrolytes and their normal range Phartoonz Electrolytes And Electrodes Functions Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrolytes are minerals with positive or negative charges like sodium, calcium, and potassium. Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Electrolytes are. Electrolytes And Electrodes Functions.
From www.youtube.com
Electrolytes and their Functions YouTube Electrolytes And Electrodes Functions Electrodes and electrolytes are integral components of electrochemical systems, each possessing unique attributes that contribute to. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Electrolytes are minerals with positive or negative charges like. Electrolytes And Electrodes Functions.
From www.researchgate.net
Schematic view of the different electrodes and electrolytes for SCs Electrolytes And Electrodes Functions Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions) across themselves and to other cells. Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Electrodes and electrolytes are integral components of. Electrolytes And Electrodes Functions.
From www.researchgate.net
Daily electrolytes requirements for adults and their function Electrolytes And Electrodes Functions Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Electrolytes are minerals with positive or negative charges like sodium, calcium, and potassium. Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their. Electrolytes And Electrodes Functions.
From healthjade.com
Electrolytes Sources and Foods High In Electrolytes Electrolytes And Electrodes Functions Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions) across themselves and to other cells. Electrolytes are minerals with positive or. Electrolytes And Electrodes Functions.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrolytes And Electrodes Functions Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to. Electrolytes And Electrodes Functions.
From med.libretexts.org
25.3A Sodium, Electrolytes, and Fluid Balance Medicine LibreTexts Electrolytes And Electrodes Functions Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrolytes are minerals with positive or negative charges like sodium, calcium, and potassium. Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating. Electrolytes And Electrodes Functions.
From www.slideserve.com
PPT Chapter 2 PowerPoint Presentation, free download ID3834624 Electrolytes And Electrodes Functions Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions) across themselves and to other cells. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrolytes are substances that have a. Electrolytes And Electrodes Functions.
From www.researchgate.net
Schematic illustration of three types of electrolytes. Download Electrolytes And Electrodes Functions Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrodes and electrolytes are integral components of electrochemical systems, each possessing unique. Electrolytes And Electrodes Functions.
From ppt-online.org
Parenteral Nutrition in Neonates презентация онлайн Electrolytes And Electrodes Functions Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Electrodes and electrolytes are integral components of electrochemical systems, each possessing unique attributes that contribute to. Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Your body needs electrolytes for brain function, muscle contractions, and managing blood. Electrolytes And Electrodes Functions.
From www.medicalkidunya.com
Electrolytes Functions, Relationships, and Imbalances medicalkidunya Electrolytes And Electrodes Functions Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrodes and electrolytes are integral components of electrochemical systems, each possessing unique attributes that contribute to. Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Electrolytes are essential for basic life functioning, such as maintaining electrical. Electrolytes And Electrodes Functions.
From www.bioticsresearch.com
ElectrolyteForte Electrolytes And Electrodes Functions Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions) across themselves and to other cells. Electrolytes are minerals that carry an electric charge when. Electrolytes And Electrodes Functions.
From mykokoronutrition.com
What happens when your body is low on electrolytes? Kokoro Nutrition Electrolytes And Electrodes Functions Electrolytes are minerals with positive or negative charges like sodium, calcium, and potassium. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use. Electrolytes And Electrodes Functions.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes Electrolytes And Electrodes Functions Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions) across themselves and to other cells. Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Electrolytes are minerals with positive or negative. Electrolytes And Electrodes Functions.
From ozziesmall.com
What are electrolytes? Ozzie Small Electrolytes And Electrodes Functions Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses,. Electrolytes And Electrodes Functions.
From www.medinmotion.com
When to add electrolytes to your hydration plan Medicine In Motion Electrolytes And Electrodes Functions Electrolytes are minerals with positive or negative charges like sodium, calcium, and potassium. Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid,. Electrolytes And Electrodes Functions.
From www.phartoonz.com
List of electrolytes and their normal range Phartoonz Electrolytes And Electrodes Functions Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions). Electrolytes And Electrodes Functions.
From www.pathwaystochemistry.com
Electrolytes and Nonelectrolytes Pathways to Chemistry Electrolytes And Electrodes Functions Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrolytes are minerals with positive or negative charges like sodium, calcium, and potassium. Electrodes and electrolytes are integral components of electrochemical systems, each possessing unique attributes that contribute to. Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in. Electrolytes And Electrodes Functions.
From studylib.net
E5 ELECTRODES AND ELECTROLYTES Electrolytes And Electrodes Functions Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells. Electrolytes And Electrodes Functions.
From www.mysportscience.com
The roles of other electrolytes Electrolytes And Electrodes Functions Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions) across themselves and to other cells. Electrolytes are minerals that carry an electric charge when. Electrolytes And Electrodes Functions.
From www.pinterest.co.uk
What Are Electrolytes and Why Are They Important? Fullscript Electrolytes And Electrodes Functions Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions) across themselves and to other cells. Electrolytes are minerals with positive or negative charges like sodium, calcium,. Electrolytes And Electrodes Functions.
From siimland.com
Full Guide On How to Get Electrolytes on Keto Diet Siim Land Electrolytes And Electrodes Functions Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions) across themselves and to other cells. Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrolytes are essential for basic life functioning, such as maintaining electrical. Electrolytes And Electrodes Functions.
From www.mysportscience.com
Electrolytes under investigation Electrolytes And Electrodes Functions Electrolytes are minerals with positive or negative charges like sodium, calcium, and potassium. Electrodes and electrolytes are integral components of electrochemical systems, each possessing unique attributes that contribute to. Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrolytes are minerals. Electrolytes And Electrodes Functions.
From www.youtube.com
Electrolytes What Are Electrolytes Functions Of Electrolytes YouTube Electrolytes And Electrodes Functions Electrodes and electrolytes are integral components of electrochemical systems, each possessing unique attributes that contribute to. Electrolytes are minerals with positive or negative charges like sodium, calcium, and potassium. Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Electrolytes are minerals that carry an electric charge when they are dissolved in a. Electrolytes And Electrodes Functions.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Electrolytes And Electrodes Functions Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses,. Electrolytes And Electrodes Functions.
From www.batterypowertips.com
What is an electrolyte? Battery Power Tips Electrolytes And Electrodes Functions Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions) across themselves and to other cells. Electrolytes are minerals with positive or negative charges like sodium, calcium, and potassium. Electrodes and electrolytes are integral components of electrochemical systems, each possessing. Electrolytes And Electrodes Functions.
From what-benefits.com
How Do Electrolytes Benefit The Body Electrolytes And Electrodes Functions Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrodes and electrolytes are integral components of electrochemical systems, each possessing unique attributes that contribute to. Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and. Electrolytes are minerals with positive or negative charges like sodium, calcium, and potassium. Electrolytes. Electrolytes And Electrodes Functions.
From saylordotorg.github.io
Electrolysis Electrolytes And Electrodes Functions Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrolytes are minerals with positive or negative charges like sodium, calcium, and potassium. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain. Electrolytes And Electrodes Functions.
From news.cancerconnect.com
Electrolyte Imbalance Overview CancerConnect Electrolytes And Electrodes Functions Electrolytes are minerals with positive or negative charges like sodium, calcium, and potassium. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain. Electrolytes And Electrodes Functions.
From www.netmeds.com
Know Why Electrolytes Are Important For You To Stay Healthy Infographic Electrolytes And Electrodes Functions Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrolytes are minerals with positive or negative charges like sodium, calcium, and potassium. Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells. Electrolytes And Electrodes Functions.
From www.slideserve.com
PPT Solutions Review Pt 2 PowerPoint Presentation, free download ID Electrolytes And Electrodes Functions Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrodes and electrolytes are integral components of electrochemical systems, each possessing unique attributes that contribute to. Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating. Electrolytes And Electrodes Functions.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Electrolytes And Electrodes Functions Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions) across themselves and to other cells. Electrodes and electrolytes are integral components of electrochemical systems, each possessing unique attributes that contribute to. Electrolytes are minerals with positive or negative charges. Electrolytes And Electrodes Functions.
From www.yourdictionary.com
Examples of Electrolytes Basic Explanation and Purpose YourDictionary Electrolytes And Electrodes Functions Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in. Your body needs electrolytes for brain function, muscle contractions, and managing blood ph. Electrolytes are important because they are what your cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses (nerve impulses, muscle contractions) across themselves. Electrolytes And Electrodes Functions.