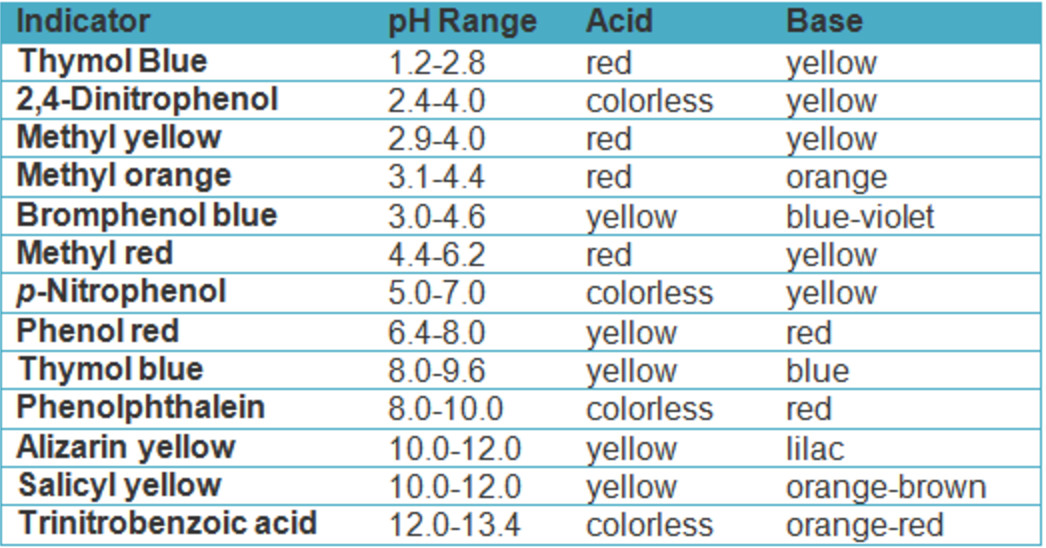
Titration Indicator Called . A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Perform and interpret titration calculations. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Both indicators change colour over a specific ph range. In many titrations, you use a chemical. For many titration reactions it is possible to find a suitable visual colour indicator that will signal the end point at, or very close to, the equivalence point. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration.
from classnotes.org.in
Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Both indicators change colour over a specific ph range. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. For many titration reactions it is possible to find a suitable visual colour indicator that will signal the end point at, or very close to, the equivalence point. In many titrations, you use a chemical. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. Perform and interpret titration calculations.
Acid Base Titration using Indicator Chemistry, Class 11, Ionic
Titration Indicator Called In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. For many titration reactions it is possible to find a suitable visual colour indicator that will signal the end point at, or very close to, the equivalence point. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. In many titrations, you use a chemical. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Perform and interpret titration calculations. Both indicators change colour over a specific ph range.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Titration Indicator Called The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Both. Titration Indicator Called.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Titration Indicator Called Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. For many titration reactions it is possible to find a suitable visual colour indicator that will signal the end point at, or very close to, the equivalence point. The volumes of acids and alkali solutions that react. Titration Indicator Called.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Titration Indicator Called The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. Both indicators change colour over a specific ph range. Perform and interpret titration calculations. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In many titrations, you use a chemical. Titration is the. Titration Indicator Called.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Indicator Called For many titration reactions it is possible to find a suitable visual colour indicator that will signal the end point at, or very close to, the equivalence point. Both indicators change colour over a specific ph range. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. The volumes of acids. Titration Indicator Called.
From www.sliderbase.com
Titration Titration Indicator Called Both indicators change colour over a specific ph range. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. In many titrations, you use a chemical.. Titration Indicator Called.
From studylib.net
The titration of an Indicator (HIn) Titration Indicator Called The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. In many titrations,. Titration Indicator Called.
From www.youtube.com
Titration Curves for High School Chemistry YouTube Titration Indicator Called A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Perform and interpret titration calculations. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Titration is. Titration Indicator Called.
From freesvg.org
Redox Titration Using Indicator Free SVG Titration Indicator Called Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. The volumes of acids and alkali solutions that react with each other can be measured by titration using. Titration Indicator Called.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Titration Indicator Called In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Perform and interpret titration calculations. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator.. Titration Indicator Called.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Indicator Called The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. For many titration. Titration Indicator Called.
From mungfali.com
Acid Base Titration Indicator Titration Indicator Called In many titrations, you use a chemical. Both indicators change colour over a specific ph range. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Perform and interpret titration calculations. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable. Titration Indicator Called.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Titration Indicator Called Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. For many titration reactions it is possible to find a suitable visual colour indicator that will signal the end point at, or very close to, the equivalence point. Both indicators change colour over a specific ph range.. Titration Indicator Called.
From www.science-revision.co.uk
Titrations Titration Indicator Called Both indicators change colour over a specific ph range. For many titration reactions it is possible to find a suitable visual colour indicator that will signal the end point at, or very close to, the equivalence point. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. The two most common. Titration Indicator Called.
From www.hoddereducationmagazines.com
Performing the perfect titration Hodder Education Magazines Titration Indicator Called Both indicators change colour over a specific ph range. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Titration is the slow addition of one solution of a known concentration (called a titrant). Titration Indicator Called.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Indicator Called In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. In many titrations, you use a chemical. Perform and interpret titration calculations. For many titration reactions it is possible to. Titration Indicator Called.
From www.slideserve.com
PPT Volumetric ANALYSIS/TITRATION PowerPoint Presentation, free Titration Indicator Called The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. In a titration, you determine an unknown concentration of a sample by adding a second reactant. Titration Indicator Called.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titration Indicator Called For many titration reactions it is possible to find a suitable visual colour indicator that will signal the end point at, or very close to, the equivalence point. In many titrations, you use a chemical. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The volumes of acids and alkali solutions that react with. Titration Indicator Called.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Indicator Called Both indicators change colour over a specific ph range. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Perform and interpret titration calculations. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. In a titration, you determine an. Titration Indicator Called.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2976284 Titration Indicator Called The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of. Titration Indicator Called.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Indicator Called A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. In many titrations, you use a chemical. The two most common indicators that are used in titrations are methyl orange and phenolphthalein.. Titration Indicator Called.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Indicator Called Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Perform and interpret titration calculations. Both indicators change colour over a specific ph range. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. A titration is a laboratory technique used to. Titration Indicator Called.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Titration Indicator Called The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. In. Titration Indicator Called.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Indicator Called Perform and interpret titration calculations. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. For many titration reactions it is possible to find a suitable visual colour indicator that will signal the end point at, or very close to, the equivalence point. In a titration, you determine an unknown concentration. Titration Indicator Called.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Titration Indicator Called A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. For many titration reactions it is possible to find a suitable visual colour indicator that will signal the end point at, or very close to, the equivalence point. Both indicators change colour over a specific ph range. Perform and interpret titration. Titration Indicator Called.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Indicator Called Perform and interpret titration calculations. For many titration reactions it is possible to find a suitable visual colour indicator that will signal the end point at, or very close to, the equivalence point. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Both indicators change colour over a specific ph. Titration Indicator Called.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titration Indicator Called For many titration reactions it is possible to find a suitable visual colour indicator that will signal the end point at, or very close to, the equivalence point. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using. Titration Indicator Called.
From pressbooks.bccampus.ca
8.7 AcidBase Titrations Chemistry for Chemical Engineers Titration Indicator Called The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. For many titration. Titration Indicator Called.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Indicator Called A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. For many titration reactions it is possible to find a suitable visual colour indicator that will signal the end point at, or very close to, the. Titration Indicator Called.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Titration Indicator Called Perform and interpret titration calculations. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. For many titration reactions it is possible to find a suitable visual colour indicator that. Titration Indicator Called.
From mungfali.com
Acid Base Titration Indicator Titration Indicator Called Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Perform. Titration Indicator Called.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Indicator Called The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. For many titration reactions it is possible to find a suitable visual colour indicator that will signal the end point at, or very close to, the equivalence point. In many titrations, you use a chemical. A titration is a. Titration Indicator Called.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Titration Indicator Called In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. In many titrations, you use a chemical. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. Perform and interpret titration calculations. Both indicators change colour over a specific ph. Titration Indicator Called.
From mungfali.com
Acid Base Titration Indicator Titration Indicator Called In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Both indicators change colour over a specific ph range. In many titrations, you use a chemical. The volumes. Titration Indicator Called.
From www.rdworldonline.com
What are titration instruments? Research & Development World Titration Indicator Called The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Both indicators change colour over a specific ph range. In many titrations, you use a chemical. Titration is the slow. Titration Indicator Called.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Indicator Called Both indicators change colour over a specific ph range. In many titrations, you use a chemical. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. A titration is a laboratory technique. Titration Indicator Called.