What Are Clock Reactions Used For . A clock reaction involves measuring the time taken for a particular amount of product to be produced by a chemical reaction for different reactant concentrations. The iodine clock reaction exists in several variations, which each involve iodine species (iodide ion, free iodine, or iodate ion) and redox. In this experiment, you prepare two simple, transparent solutions. In a typical reaction the initial rate (at t=0) doesn’t actually change over a. The length of time taken to form a small amount of product is measured. The iodine clock reaction is a favorite demonstration in chemistry classes because it has an element of drama. For this, the reaction persists as a staple of general chemistry lab demonstrations. Clock reactions are used to find the initial rate of a reaction. In this experiment, we will examine the effects of both temperature (cold and hot) and the effect of a catalyst on the rate of the. The iodine clock reaction is a classic chemistry experiment that demonstrates many basic principles of kinetics and redox chemistry. A clock reaction can be used to measure the rate of reaction in certain circumstances. In this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of the reaction with respect to iodide (n).
from chemistry.stackexchange.com
In a typical reaction the initial rate (at t=0) doesn’t actually change over a. For this, the reaction persists as a staple of general chemistry lab demonstrations. The length of time taken to form a small amount of product is measured. The iodine clock reaction is a classic chemistry experiment that demonstrates many basic principles of kinetics and redox chemistry. A clock reaction involves measuring the time taken for a particular amount of product to be produced by a chemical reaction for different reactant concentrations. The iodine clock reaction exists in several variations, which each involve iodine species (iodide ion, free iodine, or iodate ion) and redox. Clock reactions are used to find the initial rate of a reaction. In this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of the reaction with respect to iodide (n). The iodine clock reaction is a favorite demonstration in chemistry classes because it has an element of drama. In this experiment, you prepare two simple, transparent solutions.
reaction mechanism What reagents change the time of a chemical clock
What Are Clock Reactions Used For In this experiment, you prepare two simple, transparent solutions. In a typical reaction the initial rate (at t=0) doesn’t actually change over a. Clock reactions are used to find the initial rate of a reaction. In this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of the reaction with respect to iodide (n). The iodine clock reaction is a favorite demonstration in chemistry classes because it has an element of drama. The length of time taken to form a small amount of product is measured. A clock reaction can be used to measure the rate of reaction in certain circumstances. In this experiment, we will examine the effects of both temperature (cold and hot) and the effect of a catalyst on the rate of the. For this, the reaction persists as a staple of general chemistry lab demonstrations. A clock reaction involves measuring the time taken for a particular amount of product to be produced by a chemical reaction for different reactant concentrations. The iodine clock reaction is a classic chemistry experiment that demonstrates many basic principles of kinetics and redox chemistry. The iodine clock reaction exists in several variations, which each involve iodine species (iodide ion, free iodine, or iodate ion) and redox. In this experiment, you prepare two simple, transparent solutions.
From www.sciencephoto.com
Iodine Clock Reaction, 7 of 7 Stock Image C036/3755 Science Photo What Are Clock Reactions Used For Clock reactions are used to find the initial rate of a reaction. In this experiment, you prepare two simple, transparent solutions. The iodine clock reaction exists in several variations, which each involve iodine species (iodide ion, free iodine, or iodate ion) and redox. The iodine clock reaction is a classic chemistry experiment that demonstrates many basic principles of kinetics and. What Are Clock Reactions Used For.
From alucation.alugha.com
Amazing Iodine Clock Chemical Reaction alucation What Are Clock Reactions Used For In a typical reaction the initial rate (at t=0) doesn’t actually change over a. A clock reaction involves measuring the time taken for a particular amount of product to be produced by a chemical reaction for different reactant concentrations. Clock reactions are used to find the initial rate of a reaction. In this experiment, we will examine the effects of. What Are Clock Reactions Used For.
From www.youtube.com
Iodine Clock Reaction Explained (Chemistry) YouTube What Are Clock Reactions Used For Clock reactions are used to find the initial rate of a reaction. A clock reaction involves measuring the time taken for a particular amount of product to be produced by a chemical reaction for different reactant concentrations. The iodine clock reaction exists in several variations, which each involve iodine species (iodide ion, free iodine, or iodate ion) and redox. The. What Are Clock Reactions Used For.
From www.wikihow.com
How to Perform the Iodine Clock Reaction 11 Steps (with Pictures) What Are Clock Reactions Used For The iodine clock reaction exists in several variations, which each involve iodine species (iodide ion, free iodine, or iodate ion) and redox. The length of time taken to form a small amount of product is measured. In a typical reaction the initial rate (at t=0) doesn’t actually change over a. A clock reaction can be used to measure the rate. What Are Clock Reactions Used For.
From crunchchemistry.co.uk
What is a clock reaction? Crunch Chemistry What Are Clock Reactions Used For The iodine clock reaction exists in several variations, which each involve iodine species (iodide ion, free iodine, or iodate ion) and redox. In this experiment, we will examine the effects of both temperature (cold and hot) and the effect of a catalyst on the rate of the. A clock reaction can be used to measure the rate of reaction in. What Are Clock Reactions Used For.
From www.youtube.com
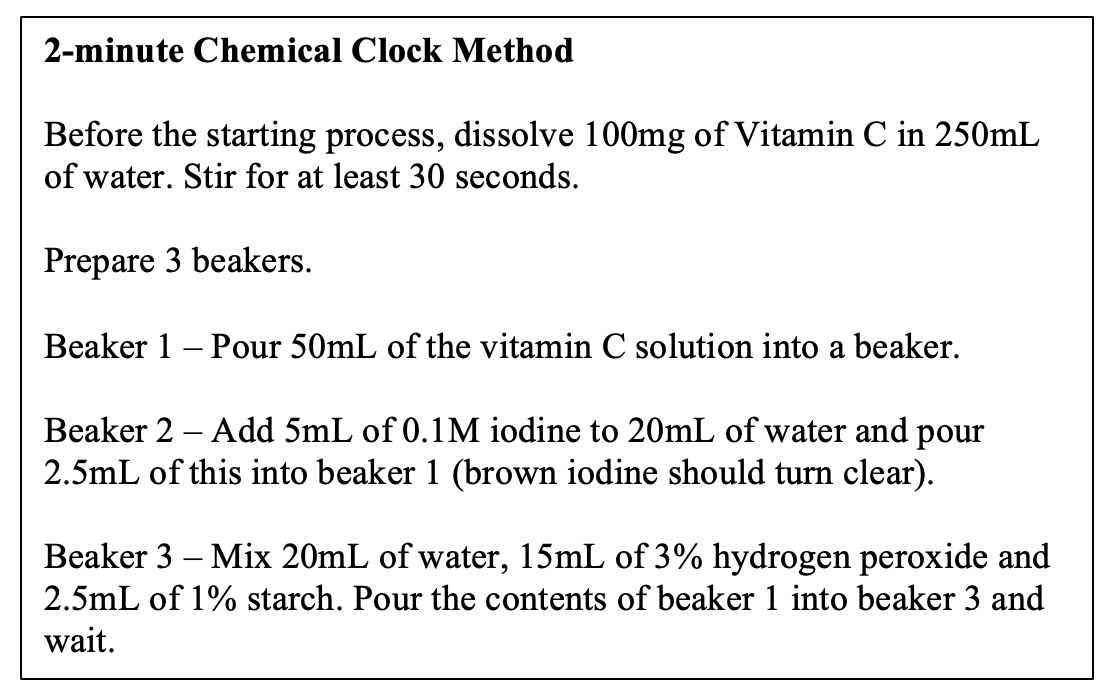
Recreating the Iodine Clock Reaction at Home with Vitamin C YouTube What Are Clock Reactions Used For The iodine clock reaction is a classic chemistry experiment that demonstrates many basic principles of kinetics and redox chemistry. In this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of the reaction with respect to iodide (n). In this experiment, you prepare two simple, transparent solutions.. What Are Clock Reactions Used For.
From studylib.net
Rates of Chemical Reactions I A Clock Reaction What Are Clock Reactions Used For For this, the reaction persists as a staple of general chemistry lab demonstrations. A clock reaction involves measuring the time taken for a particular amount of product to be produced by a chemical reaction for different reactant concentrations. A clock reaction can be used to measure the rate of reaction in certain circumstances. The length of time taken to form. What Are Clock Reactions Used For.
From www.youtube.com
How to do lab report [Exp 004] Rates of Reaction for Iodine Clock What Are Clock Reactions Used For In a typical reaction the initial rate (at t=0) doesn’t actually change over a. A clock reaction involves measuring the time taken for a particular amount of product to be produced by a chemical reaction for different reactant concentrations. The iodine clock reaction exists in several variations, which each involve iodine species (iodide ion, free iodine, or iodate ion) and. What Are Clock Reactions Used For.
From www.slideserve.com
PPT Chemical Lab The formaldehyde clock reaction PowerPoint What Are Clock Reactions Used For In this experiment, you prepare two simple, transparent solutions. The iodine clock reaction is a favorite demonstration in chemistry classes because it has an element of drama. For this, the reaction persists as a staple of general chemistry lab demonstrations. In this experiment, we will examine the effects of both temperature (cold and hot) and the effect of a catalyst. What Are Clock Reactions Used For.
From loerwzwja.blob.core.windows.net
How Does Iodine Clock Reaction Work at John Peavler blog What Are Clock Reactions Used For For this, the reaction persists as a staple of general chemistry lab demonstrations. The iodine clock reaction is a favorite demonstration in chemistry classes because it has an element of drama. In a typical reaction the initial rate (at t=0) doesn’t actually change over a. In this experiment we use the initial rate method to find the order of the. What Are Clock Reactions Used For.
From www.youtube.com
Make the Iodine Clock Reaction (Chemistry) YouTube What Are Clock Reactions Used For For this, the reaction persists as a staple of general chemistry lab demonstrations. In a typical reaction the initial rate (at t=0) doesn’t actually change over a. Clock reactions are used to find the initial rate of a reaction. The iodine clock reaction is a favorite demonstration in chemistry classes because it has an element of drama. The iodine clock. What Are Clock Reactions Used For.
From www.youtube.com
Iodine Clock Reaction Chemistry YouTube What Are Clock Reactions Used For A clock reaction can be used to measure the rate of reaction in certain circumstances. The iodine clock reaction exists in several variations, which each involve iodine species (iodide ion, free iodine, or iodate ion) and redox. In a typical reaction the initial rate (at t=0) doesn’t actually change over a. In this experiment, you prepare two simple, transparent solutions.. What Are Clock Reactions Used For.
From www.youtube.com
Clock reactions A Level chemistry practical techniques YouTube What Are Clock Reactions Used For In this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of the reaction with respect to iodide (n). In this experiment, you prepare two simple, transparent solutions. In a typical reaction the initial rate (at t=0) doesn’t actually change over a. The iodine clock reaction exists. What Are Clock Reactions Used For.
From www.youtube.com
Iodine Clock experiment explained (Grade 12 school science lab) YouTube What Are Clock Reactions Used For A clock reaction can be used to measure the rate of reaction in certain circumstances. In this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of the reaction with respect to iodide (n). The iodine clock reaction is a favorite demonstration in chemistry classes because it. What Are Clock Reactions Used For.
From www.youtube.com
Iodine Clock Experiment (Clock Reactions Alevel IB Chemistry) YouTube What Are Clock Reactions Used For In this experiment, we will examine the effects of both temperature (cold and hot) and the effect of a catalyst on the rate of the. A clock reaction involves measuring the time taken for a particular amount of product to be produced by a chemical reaction for different reactant concentrations. The iodine clock reaction is a favorite demonstration in chemistry. What Are Clock Reactions Used For.
From crunchchemistry.co.uk
What is a clock reaction? Crunch Chemistry What Are Clock Reactions Used For The iodine clock reaction exists in several variations, which each involve iodine species (iodide ion, free iodine, or iodate ion) and redox. In a typical reaction the initial rate (at t=0) doesn’t actually change over a. The length of time taken to form a small amount of product is measured. In this experiment we use the initial rate method to. What Are Clock Reactions Used For.
From www.studocu.com
Clock Reactions Notes ⏰ Clock Reactions → In a clock reaction What Are Clock Reactions Used For In this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of the reaction with respect to iodide (n). A clock reaction can be used to measure the rate of reaction in certain circumstances. For this, the reaction persists as a staple of general chemistry lab demonstrations.. What Are Clock Reactions Used For.
From iteachly.com
The Iodine Clock Reaction Lab ⋆ Chemistry What Are Clock Reactions Used For The iodine clock reaction is a classic chemistry experiment that demonstrates many basic principles of kinetics and redox chemistry. In this experiment, you prepare two simple, transparent solutions. In a typical reaction the initial rate (at t=0) doesn’t actually change over a. For this, the reaction persists as a staple of general chemistry lab demonstrations. Clock reactions are used to. What Are Clock Reactions Used For.
From www.slideserve.com
PPT Chemical Lab The formaldehyde clock reaction PowerPoint What Are Clock Reactions Used For For this, the reaction persists as a staple of general chemistry lab demonstrations. In a typical reaction the initial rate (at t=0) doesn’t actually change over a. The iodine clock reaction is a classic chemistry experiment that demonstrates many basic principles of kinetics and redox chemistry. A clock reaction can be used to measure the rate of reaction in certain. What Are Clock Reactions Used For.
From www.slideserve.com
PPT Chapter 13 Chemical PowerPoint Presentation, free What Are Clock Reactions Used For A clock reaction can be used to measure the rate of reaction in certain circumstances. In a typical reaction the initial rate (at t=0) doesn’t actually change over a. Clock reactions are used to find the initial rate of a reaction. In this experiment, we will examine the effects of both temperature (cold and hot) and the effect of a. What Are Clock Reactions Used For.
From www.studypool.com
SOLUTION Powerpoint lab 1 of the iodine clock reaction What Are Clock Reactions Used For The iodine clock reaction exists in several variations, which each involve iodine species (iodide ion, free iodine, or iodate ion) and redox. The length of time taken to form a small amount of product is measured. In this experiment, we will examine the effects of both temperature (cold and hot) and the effect of a catalyst on the rate of. What Are Clock Reactions Used For.
From www.youtube.com
Chemical Clock Reaction YouTube What Are Clock Reactions Used For In this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of the reaction with respect to iodide (n). Clock reactions are used to find the initial rate of a reaction. A clock reaction can be used to measure the rate of reaction in certain circumstances. In. What Are Clock Reactions Used For.
From www.carolina.com
Iodine Clock Reaction Digital Resources What Are Clock Reactions Used For In this experiment, we will examine the effects of both temperature (cold and hot) and the effect of a catalyst on the rate of the. For this, the reaction persists as a staple of general chemistry lab demonstrations. A clock reaction can be used to measure the rate of reaction in certain circumstances. In this experiment, you prepare two simple,. What Are Clock Reactions Used For.
From www.chegg.com
Introduction In a "clock" reaction, there is no What Are Clock Reactions Used For Clock reactions are used to find the initial rate of a reaction. In a typical reaction the initial rate (at t=0) doesn’t actually change over a. A clock reaction involves measuring the time taken for a particular amount of product to be produced by a chemical reaction for different reactant concentrations. The iodine clock reaction is a favorite demonstration in. What Are Clock Reactions Used For.
From www.chemistrystudent.com
Clock Reactions and Iodine Clock Reaction (ALevel) ChemistryStudent What Are Clock Reactions Used For In this experiment, we will examine the effects of both temperature (cold and hot) and the effect of a catalyst on the rate of the. A clock reaction can be used to measure the rate of reaction in certain circumstances. The length of time taken to form a small amount of product is measured. For this, the reaction persists as. What Are Clock Reactions Used For.
From chemistry.stackexchange.com
reaction mechanism What reagents change the time of a chemical clock What Are Clock Reactions Used For A clock reaction involves measuring the time taken for a particular amount of product to be produced by a chemical reaction for different reactant concentrations. The length of time taken to form a small amount of product is measured. The iodine clock reaction is a favorite demonstration in chemistry classes because it has an element of drama. In this experiment,. What Are Clock Reactions Used For.
From www.youtube.com
Iodine Clock Reaction YouTube What Are Clock Reactions Used For A clock reaction involves measuring the time taken for a particular amount of product to be produced by a chemical reaction for different reactant concentrations. The iodine clock reaction exists in several variations, which each involve iodine species (iodide ion, free iodine, or iodate ion) and redox. A clock reaction can be used to measure the rate of reaction in. What Are Clock Reactions Used For.
From www.slideserve.com
PPT Iodine Clock Reaction PowerPoint Presentation, free download ID What Are Clock Reactions Used For In this experiment, you prepare two simple, transparent solutions. In this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of the reaction with respect to iodide (n). The iodine clock reaction is a classic chemistry experiment that demonstrates many basic principles of kinetics and redox chemistry.. What Are Clock Reactions Used For.
From www.slideserve.com
PPT Collision Theory and the Rate of Reaction PowerPoint Presentation What Are Clock Reactions Used For For this, the reaction persists as a staple of general chemistry lab demonstrations. In this experiment, we will examine the effects of both temperature (cold and hot) and the effect of a catalyst on the rate of the. In this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and. What Are Clock Reactions Used For.
From www.youtube.com
The Clock Reaction YouTube What Are Clock Reactions Used For In this experiment, we will examine the effects of both temperature (cold and hot) and the effect of a catalyst on the rate of the. A clock reaction involves measuring the time taken for a particular amount of product to be produced by a chemical reaction for different reactant concentrations. The length of time taken to form a small amount. What Are Clock Reactions Used For.
From www.studypool.com
SOLUTION Powerpoint lab 1 of the iodine clock reaction What Are Clock Reactions Used For The iodine clock reaction exists in several variations, which each involve iodine species (iodide ion, free iodine, or iodate ion) and redox. In this experiment, you prepare two simple, transparent solutions. In a typical reaction the initial rate (at t=0) doesn’t actually change over a. Clock reactions are used to find the initial rate of a reaction. In this experiment,. What Are Clock Reactions Used For.
From www.youtube.com
Iodine Clock Reaction Information YouTube What Are Clock Reactions Used For A clock reaction involves measuring the time taken for a particular amount of product to be produced by a chemical reaction for different reactant concentrations. The length of time taken to form a small amount of product is measured. In a typical reaction the initial rate (at t=0) doesn’t actually change over a. In this experiment, we will examine the. What Are Clock Reactions Used For.
From rosagoodue.blogspot.com
The Endpoint of the Clock Reaction for This Experiment Is the What Are Clock Reactions Used For In this experiment we use the initial rate method to find the order of the reaction with respect to persulfate (m) and the order of the reaction with respect to iodide (n). The iodine clock reaction is a favorite demonstration in chemistry classes because it has an element of drama. A clock reaction involves measuring the time taken for a. What Are Clock Reactions Used For.
From www.youtube.com
of a Clock Reaction Sample Data YouTube What Are Clock Reactions Used For The iodine clock reaction is a classic chemistry experiment that demonstrates many basic principles of kinetics and redox chemistry. In this experiment, we will examine the effects of both temperature (cold and hot) and the effect of a catalyst on the rate of the. Clock reactions are used to find the initial rate of a reaction. A clock reaction can. What Are Clock Reactions Used For.
From studylib.net
Reaction The Iodine Clock Reaction What Are Clock Reactions Used For For this, the reaction persists as a staple of general chemistry lab demonstrations. A clock reaction involves measuring the time taken for a particular amount of product to be produced by a chemical reaction for different reactant concentrations. The length of time taken to form a small amount of product is measured. The iodine clock reaction is a classic chemistry. What Are Clock Reactions Used For.