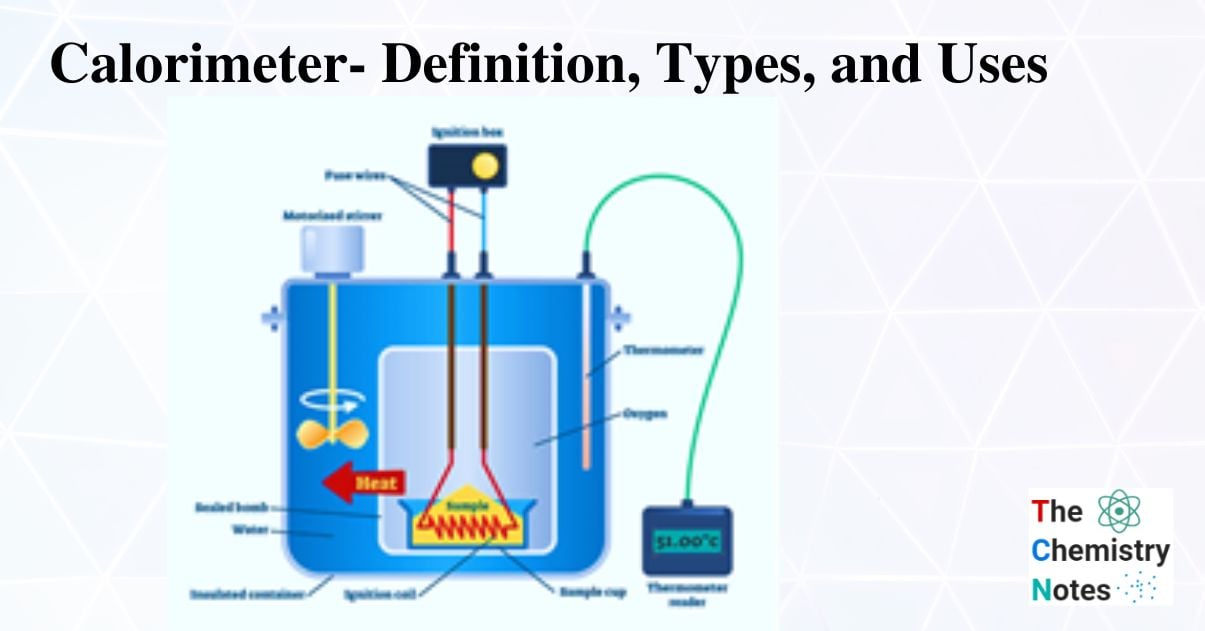
Calorimeters Are Made Of Which Of The Following . A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used for heat measurements necessary for calorimetry. From the principle of calorimetry, the heat lost by a hot body is equal to heat gained. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. For example, when an exothermic reaction occurs in solution in a calorimeter, the. It mainly consists of a metallic vessel made of materials which are good. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure. The instrumentation of a calorimeter typically includes the following components:
from scienceinfo.com
The instrumentation of a calorimeter typically includes the following components: Calorimetry is used to measure. From the principle of calorimetry, the heat lost by a hot body is equal to heat gained. For example, when an exothermic reaction occurs in solution in a calorimeter, the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. It mainly consists of a metallic vessel made of materials which are good. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used for heat measurements necessary for calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
Calorimeter Definition, Types and Uses
Calorimeters Are Made Of Which Of The Following One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The instrumentation of a calorimeter typically includes the following components: One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. It mainly consists of a metallic vessel made of materials which are good. Calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is used to measure. A calorimeter is a device used for heat measurements necessary for calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. From the principle of calorimetry, the heat lost by a hot body is equal to heat gained.
From www.translateen.com
Use "Bomb Calorimeter" In A Sentence Calorimeters Are Made Of Which Of The Following A calorimeter is a device used for heat measurements necessary for calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the. It mainly consists of a metallic vessel made of materials which are good. From the principle of calorimetry, the heat lost by a hot body is equal to heat gained. A calorimeter is a device. Calorimeters Are Made Of Which Of The Following.
From browsegrades.net
Student Exploration Calorimetry Lab Vocabulary calorie, calorimeter Calorimeters Are Made Of Which Of The Following For example, when an exothermic reaction occurs in solution in a calorimeter, the. From the principle of calorimetry, the heat lost by a hot body is equal to heat gained. The instrumentation of a calorimeter typically includes the following components: One technique we can use to measure the amount of heat involved in a chemical or physical process is known. Calorimeters Are Made Of Which Of The Following.
From www.alamy.com
Illustration depicting Rumford's calorimeter used to determine the Calorimeters Are Made Of Which Of The Following It mainly consists of a metallic vessel made of materials which are good. For example, when an exothermic reaction occurs in solution in a calorimeter, the. The instrumentation of a calorimeter typically includes the following components: A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure.. Calorimeters Are Made Of Which Of The Following.
From www.numerade.com
SOLVED Use the References to access important values if needed for Calorimeters Are Made Of Which Of The Following One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. It mainly consists of a metallic vessel made of materials which are good. The instrumentation of a calorimeter typically includes the following components: From the principle of calorimetry, the heat lost by a hot body is equal to. Calorimeters Are Made Of Which Of The Following.
From www.youtube.com
050 Calorimetry YouTube Calorimeters Are Made Of Which Of The Following Calorimetry is used to measure. For example, when an exothermic reaction occurs in solution in a calorimeter, the. From the principle of calorimetry, the heat lost by a hot body is equal to heat gained. The instrumentation of a calorimeter typically includes the following components: A calorimeter is a device used to measure the amount of heat involved in a. Calorimeters Are Made Of Which Of The Following.
From www.animalia-life.club
Calorimeter Diagram Calorimeters Are Made Of Which Of The Following It mainly consists of a metallic vessel made of materials which are good. For example, when an exothermic reaction occurs in solution in a calorimeter, the. The instrumentation of a calorimeter typically includes the following components: For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of. Calorimeters Are Made Of Which Of The Following.
From punchlistzero.com
Is Heat a State Function? Punchlist Zero Calorimeters Are Made Of Which Of The Following Calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is used to measure. From the principle of calorimetry, the heat lost by a hot body is equal to heat gained. One technique we can use to measure the amount of. Calorimeters Are Made Of Which Of The Following.
From courses.lumenlearning.com
Calorimetry CHEM 1305 Introductory Chemistry Calorimeters Are Made Of Which Of The Following A calorimeter is a device used for heat measurements necessary for calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimeters are designed. Calorimeters Are Made Of Which Of The Following.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimeters Are Made Of Which Of The Following From the principle of calorimetry, the heat lost by a hot body is equal to heat gained. For example, when an exothermic reaction occurs in solution in a calorimeter, the. The instrumentation of a calorimeter typically includes the following components: Calorimetry is used to measure. Calorimeters are designed to minimize energy exchange between the system being studied and its surroundings.. Calorimeters Are Made Of Which Of The Following.
From www.slideserve.com
PPT a “ Calorimeter ” PowerPoint Presentation, free download ID7050684 Calorimeters Are Made Of Which Of The Following Calorimetry is used to measure. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used for heat measurements necessary for calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter. Calorimeters Are Made Of Which Of The Following.
From www.bartleby.com
Answered Suppose you use a coffee cup… bartleby Calorimeters Are Made Of Which Of The Following Calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimetry is used to measure. For example, when an exothermic reaction. Calorimeters Are Made Of Which Of The Following.
From chemlab.truman.edu
Parr 1341 Bomb Calorimeter Chem Lab Calorimeters Are Made Of Which Of The Following Calorimetry is used to measure. A calorimeter is a device used for heat measurements necessary for calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimeters are. Calorimeters Are Made Of Which Of The Following.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimeters Are Made Of Which Of The Following For example, when an exothermic reaction occurs in solution in a calorimeter, the. From the principle of calorimetry, the heat lost by a hot body is equal to heat gained. The instrumentation of a calorimeter typically includes the following components: A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A. Calorimeters Are Made Of Which Of The Following.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeters Are Made Of Which Of The Following A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The instrumentation of a calorimeter typically includes the following components: It mainly consists of a metallic vessel made of materials which are good. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimeters are designed to minimize. Calorimeters Are Made Of Which Of The Following.
From www.scienceandmathwithmrslau.com
Calorimeter and Heat Exchange Chemistry Doodle Diagrams Store Calorimeters Are Made Of Which Of The Following A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It mainly consists of a metallic vessel made of materials which are good. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. From the principle of calorimetry, the. Calorimeters Are Made Of Which Of The Following.
From users.highland.edu
Calorimetry Calorimeters Are Made Of Which Of The Following Calorimetry is used to measure. The instrumentation of a calorimeter typically includes the following components: A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. From the principle of calorimetry, the heat lost by a hot body is equal to heat gained. A calorimeter is a device used to measure the. Calorimeters Are Made Of Which Of The Following.
From www.hanlin.com
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:3.1.2 Calorimetry Calorimeters Are Made Of Which Of The Following The instrumentation of a calorimeter typically includes the following components: It mainly consists of a metallic vessel made of materials which are good. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. Calorimetry is used to measure. A calorimeter is a. Calorimeters Are Made Of Which Of The Following.
From slideplayer.com
Prospects for Future measurement of GPDs using COMPASS at CERN ppt Calorimeters Are Made Of Which Of The Following The instrumentation of a calorimeter typically includes the following components: It mainly consists of a metallic vessel made of materials which are good. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure. For example, when an exothermic reaction occurs in solution in a calorimeter, the.. Calorimeters Are Made Of Which Of The Following.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimeters Are Made Of Which Of The Following For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is used to measure. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. From. Calorimeters Are Made Of Which Of The Following.
From www.chegg.com
Solved Make two calorimeters; each one made by nesting two Calorimeters Are Made Of Which Of The Following A calorimeter is a device used for heat measurements necessary for calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the. From the principle of calorimetry, the heat lost by a hot body is equal to heat gained. Calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. A calorimeter is. Calorimeters Are Made Of Which Of The Following.
From www.chegg.com
Solved Which of the following does NOT contribute to the Calorimeters Are Made Of Which Of The Following A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. From the principle of calorimetry, the heat lost by a hot body is equal to heat gained. Calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. Calorimetry is used to measure. It mainly consists of. Calorimeters Are Made Of Which Of The Following.
From www.chegg.com
Solved Which of the following is NOT a correct assumption in Calorimeters Are Made Of Which Of The Following A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimetry is used to measure. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It mainly consists of a metallic vessel made of materials which are good. Calorimeters are designed to minimize energy exchange between the system being. Calorimeters Are Made Of Which Of The Following.
From surfguppy.com
Enthalpy Surfguppy Chemistry made easy visual learning Calorimeters Are Made Of Which Of The Following A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It mainly consists of a metallic vessel made of materials which are good. Calorimetry is used to measure. From the principle of calorimetry, the heat lost by a hot body is equal to heat gained. For example, when an exothermic reaction. Calorimeters Are Made Of Which Of The Following.
From www.chegg.com
Solved Which of the following does NOT contribute to the Calorimeters Are Made Of Which Of The Following Calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. The instrumentation of a calorimeter typically includes the following components: From the principle of calorimetry, the heat lost by a hot body is equal to heat gained. For example, when an exothermic reaction occurs in solution in a calorimeter, the. It mainly consists of a. Calorimeters Are Made Of Which Of The Following.
From www.youtube.com
How to Draw a Calorimeter Step by Step Drawing Tutorial YouTube Calorimeters Are Made Of Which Of The Following One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimeters are designed to minimize energy exchange. Calorimeters Are Made Of Which Of The Following.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimeters Are Made Of Which Of The Following A calorimeter is a device used for heat measurements necessary for calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It mainly consists of a metallic vessel made of materials which are good. From the principle of calorimetry, the heat lost by a hot body is equal to heat. Calorimeters Are Made Of Which Of The Following.
From chem.libretexts.org
9.5 Calorimetry Chemistry LibreTexts Calorimeters Are Made Of Which Of The Following For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used for heat measurements necessary for calorimetry. It mainly consists of a metallic vessel made of materials which are good. Calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. For example, when an exothermic reaction occurs. Calorimeters Are Made Of Which Of The Following.
From saylordotorg.github.io
Calorimetry Calorimeters Are Made Of Which Of The Following The instrumentation of a calorimeter typically includes the following components: A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. From the principle of calorimetry, the heat lost by. Calorimeters Are Made Of Which Of The Following.
From www.pinterest.com.au
Pin on Science Friday Calorimeters Are Made Of Which Of The Following For example, when an exothermic reaction occurs in solution in a calorimeter, the. It mainly consists of a metallic vessel made of materials which are good. From the principle of calorimetry, the heat lost by a hot body is equal to heat gained. Calorimetry is used to measure. For example, when an exothermic reaction occurs in solution in a calorimeter,. Calorimeters Are Made Of Which Of The Following.
From www.chegg.com
Chemistry Archive January 13, 2019 Calorimeters Are Made Of Which Of The Following The instrumentation of a calorimeter typically includes the following components: For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device. Calorimeters Are Made Of Which Of The Following.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimeters Are Made Of Which Of The Following Calorimetry is used to measure. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in. Calorimeters Are Made Of Which Of The Following.
From www.tescaglobal.com
Digital Bomb Calorimeter Calorimeters Are Made Of Which Of The Following For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is used to measure. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. From the principle of calorimetry, the heat lost by a hot body is equal to heat gained. A calorimeter is a device used. Calorimeters Are Made Of Which Of The Following.
From huksefluxusa.com
What is a Calorimeter? — HuksefluxUSA Calorimeters Are Made Of Which Of The Following For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. One technique we can use to measure the amount of heat involved in. Calorimeters Are Made Of Which Of The Following.
From deon-has-edwards.blogspot.com
Heat Capacity of Calorimeter DeonhasEdwards Calorimeters Are Made Of Which Of The Following For example, when an exothermic reaction occurs in solution in a calorimeter, the. The instrumentation of a calorimeter typically includes the following components: A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat involved in a chemical or physical process. Calorimeters Are Made Of Which Of The Following.
From slideplayer.com
Top Quark Production at the Large Hadron Collider ppt download Calorimeters Are Made Of Which Of The Following A calorimeter is a device used for heat measurements necessary for calorimetry. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. The instrumentation of. Calorimeters Are Made Of Which Of The Following.