Surface Tension Of Water Glass Interface . We describe in great detail the. A publisher correction to this article was published on 07 august 2018. In this paper, we study drop spreading phenomena on a glass surface experimentally. The answer lies in surface tension. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. This article has been updated.
from blog.iglcoatings.com
In this paper, we study drop spreading phenomena on a glass surface experimentally. A publisher correction to this article was published on 07 august 2018. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. The answer lies in surface tension. We describe in great detail the. For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. This article has been updated.
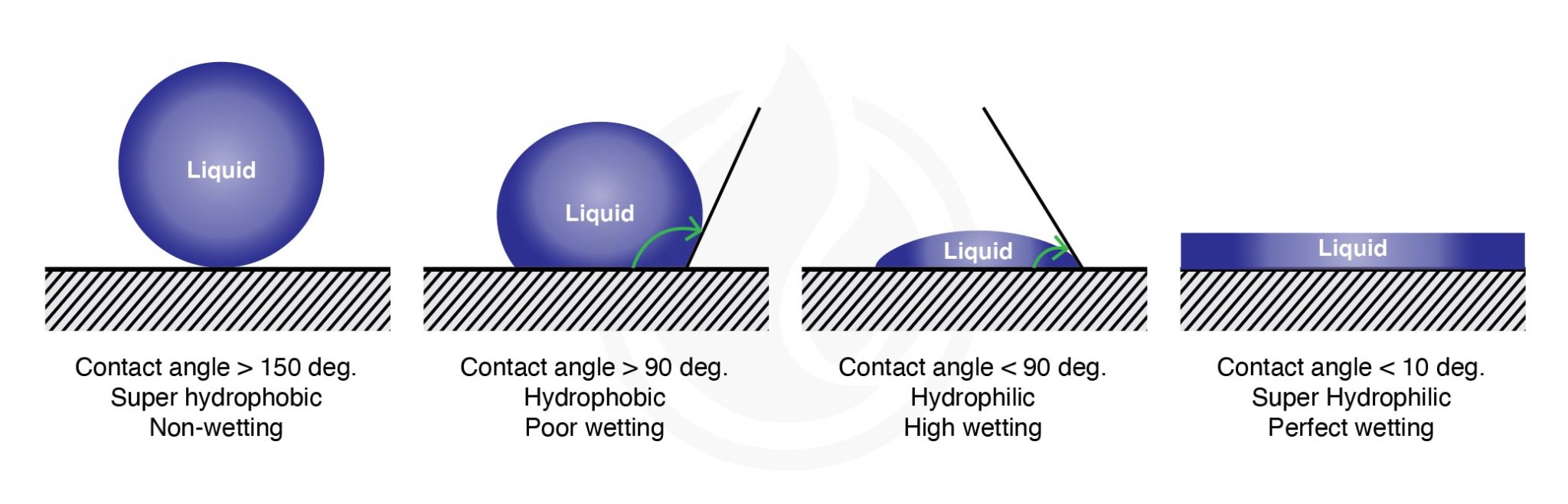
The Science of Hydrophobicity IGL Coatings Blog
Surface Tension Of Water Glass Interface In this paper, we study drop spreading phenomena on a glass surface experimentally. We describe in great detail the. A publisher correction to this article was published on 07 august 2018. The answer lies in surface tension. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. In this paper, we study drop spreading phenomena on a glass surface experimentally. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. This article has been updated.
From statdreams.weebly.com
statdreams Blog Surface Tension Of Water Glass Interface A publisher correction to this article was published on 07 august 2018. We describe in great detail the. The answer lies in surface tension. For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. In this paper, we study drop spreading phenomena on a glass surface experimentally. This article has been. Surface Tension Of Water Glass Interface.
From www.surfi.mtu.edu
Contact Angles in Flotation SURFI Surface Tension Of Water Glass Interface Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. A publisher correction to this article was published on 07 august 2018. For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. In this paper, we study drop spreading phenomena. Surface Tension Of Water Glass Interface.
From www.youtube.com
What are the application of surface tension? YouTube Surface Tension Of Water Glass Interface We describe in great detail the. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. The answer lies in surface tension. A publisher. Surface Tension Of Water Glass Interface.
From www.youtube.com
The Sci Guys Science at Home SE3 EP4 Water Glass Surface Tension Surface Tension Of Water Glass Interface We describe in great detail the. In this paper, we study drop spreading phenomena on a glass surface experimentally. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water. Surface Tension Of Water Glass Interface.
From www.youtube.com
To study surface tension of water by capillary rise method. YouTube Surface Tension Of Water Glass Interface In this paper, we study drop spreading phenomena on a glass surface experimentally. This article has been updated. We describe in great detail the. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. For instance, the surface tension at a water/air interface is significantly. Surface Tension Of Water Glass Interface.
From funsizephysics.com
What is Surface Tension? FunsizePhysics Surface Tension Of Water Glass Interface For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. We describe in great detail the. The answer lies in surface tension. In this paper, we study drop spreading phenomena on a glass surface experimentally. This article has been updated. Whether dissipative terms are relevant to periodic surface stresses at the. Surface Tension Of Water Glass Interface.
From www.flickr.com
Surface Tension A glass of water about to spill. That is t… Flickr Surface Tension Of Water Glass Interface Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water. Surface Tension Of Water Glass Interface.
From www.youtube.com
Numerical Capillary Rise or fall due to surface tension Hindi Surface Tension Of Water Glass Interface Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. This article has been updated. We describe in great detail the. A publisher correction to this article was published on 07 august 2018. The answer lies in surface tension. Whether dissipative terms are relevant to. Surface Tension Of Water Glass Interface.
From www.toppr.com
The lower end of a capillary tube of radius 1mm is dipped vertically Surface Tension Of Water Glass Interface A publisher correction to this article was published on 07 august 2018. The answer lies in surface tension. For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. In this. Surface Tension Of Water Glass Interface.
From www.researchgate.net
Schematic illustration of standard methods of surface tension Surface Tension Of Water Glass Interface This article has been updated. We describe in great detail the. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. The answer lies in surface tension. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger. Surface Tension Of Water Glass Interface.
From studyfullirene.z21.web.core.windows.net
Example Of Surface Tension For Kids Surface Tension Of Water Glass Interface For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. The answer lies in surface tension. We describe in great detail the. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. Whether dissipative terms are. Surface Tension Of Water Glass Interface.
From www.youtube.com
Surface and Interfacial Tension (1/4) Physical Pharmacy GPAT NIPER BITS Surface Tension Of Water Glass Interface The answer lies in surface tension. We describe in great detail the. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. In this paper, we study drop spreading phenomena on a glass surface experimentally. Whether dissipative terms are relevant to periodic surface stresses at. Surface Tension Of Water Glass Interface.
From ar.inspiredpencil.com
Surface Tension Of Water Molecules Surface Tension Of Water Glass Interface Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. This article has been updated. We describe in great detail the. A publisher correction. Surface Tension Of Water Glass Interface.
From www.aiophotoz.com
Surface Tension Of Water Science Experiments For Kids Images and Surface Tension Of Water Glass Interface Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. A publisher correction to this article was published on 07 august 2018. The answer lies in surface tension. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is. Surface Tension Of Water Glass Interface.
From www.numerade.com
SOLVED O.lmm Capillary Convex meniscus Hg bulk surface level lllmm Surface Tension Of Water Glass Interface In this paper, we study drop spreading phenomena on a glass surface experimentally. For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. This article has been updated. A publisher correction to this article was published on 07 august 2018. We describe in great detail the. The answer lies in surface. Surface Tension Of Water Glass Interface.
From www.kruss-scientific.com
Wilhelmy plate method KRÜSS Scientific Surface Tension Of Water Glass Interface The answer lies in surface tension. We describe in great detail the. A publisher correction to this article was published on 07 august 2018. This article has been updated. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. For instance, the surface tension at a water/air interface is. Surface Tension Of Water Glass Interface.
From exosaopkm.blob.core.windows.net
Surface TensionLiquidVapour Interface at Edward Sanders blog Surface Tension Of Water Glass Interface We describe in great detail the. The answer lies in surface tension. This article has been updated. For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. A publisher correction. Surface Tension Of Water Glass Interface.
From www.biolinscientific.com
Surface tension of water Why is it so high? Surface Tension Of Water Glass Interface A publisher correction to this article was published on 07 august 2018. We describe in great detail the. For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. This article has been updated. The answer lies in surface tension. Whether dissipative terms are relevant to periodic surface stresses at the pure. Surface Tension Of Water Glass Interface.
From brunofuga.adv.br
Surface Tension Of Water Why Is It So High?, 43 OFF Surface Tension Of Water Glass Interface For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. The answer lies in surface tension. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. This article has been updated. Whether dissipative terms are relevant. Surface Tension Of Water Glass Interface.
From www.researchgate.net
Surface Tension at the air/water interface of (A) FB 1 g l − 1 Surface Tension Of Water Glass Interface The answer lies in surface tension. A publisher correction to this article was published on 07 august 2018. This article has been updated. In this paper, we study drop spreading phenomena on a glass surface experimentally. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. Because intermolecular forces. Surface Tension Of Water Glass Interface.
From blog.iglcoatings.com
The Science of Hydrophobicity IGL Coatings Blog Surface Tension Of Water Glass Interface In this paper, we study drop spreading phenomena on a glass surface experimentally. We describe in great detail the. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. This article has been updated. A publisher correction to this article was published on 07 august. Surface Tension Of Water Glass Interface.
From www.researchgate.net
(A) Dynamic interfacial tension of the water−silicone oil interface at Surface Tension Of Water Glass Interface We describe in great detail the. For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. A publisher correction to this article was published on 07 august 2018. In this. Surface Tension Of Water Glass Interface.
From lexica.art
Lexica Breaking the surface tension of water, photograph Surface Tension Of Water Glass Interface Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. We describe in great detail the. This article has been updated. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. For instance, the. Surface Tension Of Water Glass Interface.
From www.chegg.com
Solved If the surface tension of water is 0.0728 what is the Surface Tension Of Water Glass Interface A publisher correction to this article was published on 07 august 2018. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. The answer. Surface Tension Of Water Glass Interface.
From fity.club
Adhesion Water Surface Tension Of Water Glass Interface For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. The answer lies in surface tension. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. A publisher correction to this article was published on 07. Surface Tension Of Water Glass Interface.
From readchemistry.com
Determination of Surface Tension Read Chemistry Surface Tension Of Water Glass Interface Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. This article has been updated. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. The answer lies in surface tension. We describe in. Surface Tension Of Water Glass Interface.
From www.chegg.com
Solved Question No. 1. (10 marks) A glass capillary tube of Surface Tension Of Water Glass Interface Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. A publisher correction to this article was published on 07 august 2018. For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. The answer lies in. Surface Tension Of Water Glass Interface.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Surface Tension Of Water Glass Interface We describe in great detail the. A publisher correction to this article was published on 07 august 2018. The answer lies in surface tension. This article has been updated. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. In this paper, we study drop spreading phenomena on a. Surface Tension Of Water Glass Interface.
From materialmcgheeferried.z21.web.core.windows.net
How To Explain Surface Tension Surface Tension Of Water Glass Interface Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. For instance, the surface tension at a water/air interface is significantly deceased in the. Surface Tension Of Water Glass Interface.
From pressbooks.uiowa.edu
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary Surface Tension Of Water Glass Interface This article has been updated. In this paper, we study drop spreading phenomena on a glass surface experimentally. The answer lies in surface tension. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. A publisher correction to this article was published on 07 august 2018. We describe in. Surface Tension Of Water Glass Interface.
From www.youtube.com
Difference between surface and interface surface tension and Surface Tension Of Water Glass Interface In this paper, we study drop spreading phenomena on a glass surface experimentally. The answer lies in surface tension. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. A publisher correction to this article was published on 07 august 2018. Whether dissipative terms are. Surface Tension Of Water Glass Interface.
From blog.iglcoatings.com
The Science of Hydrophobicity IGL Coatings Blog Surface Tension Of Water Glass Interface For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. In this paper, we study drop spreading phenomena on a glass surface experimentally. We describe in great detail the. This article has been updated. A publisher correction to this article was published on 07 august 2018. The answer lies in surface. Surface Tension Of Water Glass Interface.
From www.writework.com
Surface Tension WriteWork Surface Tension Of Water Glass Interface Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. We describe in great detail the. In this paper, we study drop spreading phenomena on a glass surface experimentally. For instance, the surface tension at a water/air interface is significantly deceased in the presence of adsorbed soap molecules. Because. Surface Tension Of Water Glass Interface.
From ar.inspiredpencil.com
Surface Tension Of Water Molecules Surface Tension Of Water Glass Interface The answer lies in surface tension. We describe in great detail the. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. In this. Surface Tension Of Water Glass Interface.
From dxoyyqhyx.blob.core.windows.net
Water Surface Tension Meaning at Mary Tully blog Surface Tension Of Water Glass Interface The answer lies in surface tension. Because intermolecular forces between water molecules are due to hydrogen bonds and these are high energy, surface tension for water is larger than many other. Whether dissipative terms are relevant to periodic surface stresses at the pure water/air interface is addressed by valuable new measurements on. In this paper, we study drop spreading phenomena. Surface Tension Of Water Glass Interface.