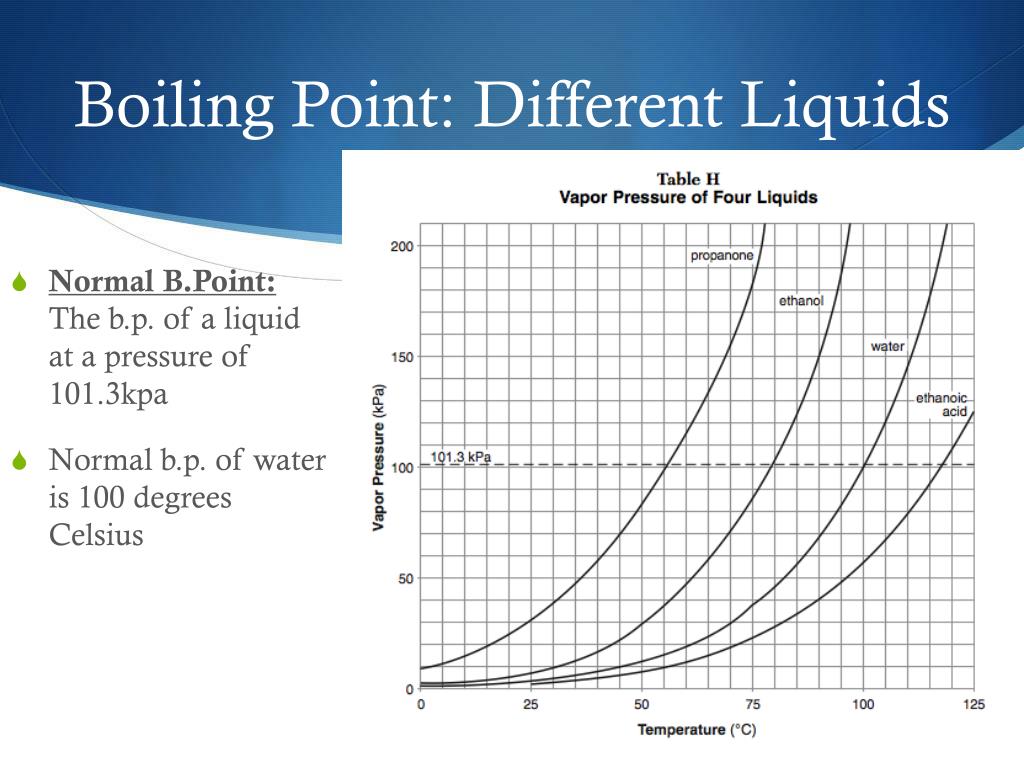
What Raises The Boiling Point Of A Liquid . A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Normally when we boil a liquid, we do so at atmospheric pressure. This is the boiling point which is usually quoted in chemical literature. As the altitude increases, the boiling. In an open system this is called atmospheric pressure. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; The pressure of gas above a liquid affects the boiling point. As the altitude increases, the boiling point decreases. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Under this condition, addition of heat results in the transformation of the liquid into its vapor without raising the temperature. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure.
from www.slideserve.com
As the altitude increases, the boiling. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. This is the boiling point which is usually quoted in chemical literature. Normally when we boil a liquid, we do so at atmospheric pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; In an open system this is called atmospheric pressure. Under this condition, addition of heat results in the transformation of the liquid into its vapor without raising the temperature.
PPT Chapter 13 continued… PowerPoint Presentation, free download ID
What Raises The Boiling Point Of A Liquid Under this condition, addition of heat results in the transformation of the liquid into its vapor without raising the temperature. Under this condition, addition of heat results in the transformation of the liquid into its vapor without raising the temperature. As the altitude increases, the boiling point decreases. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The pressure of gas above a liquid affects the boiling point. In an open system this is called atmospheric pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; As the altitude increases, the boiling. This is the boiling point which is usually quoted in chemical literature. Normally when we boil a liquid, we do so at atmospheric pressure.
From www.slideserve.com
PPT Chapter 12 Solutions PowerPoint Presentation, free download ID What Raises The Boiling Point Of A Liquid In an open system this is called atmospheric pressure. This is the boiling point which is usually quoted in chemical literature. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; As the altitude increases, the boiling point decreases. As the altitude increases, the. What Raises The Boiling Point Of A Liquid.
From www.numerade.com
SOLVED The boiling point of a liquid increases as the pressure above What Raises The Boiling Point Of A Liquid This is the boiling point which is usually quoted in chemical literature. As the altitude increases, the boiling point decreases. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Under this condition, addition of heat results in the transformation of the liquid into its vapor without raising the temperature.. What Raises The Boiling Point Of A Liquid.
From www.slideserve.com
PPT Ch. 13 States of Matter PowerPoint Presentation, free download What Raises The Boiling Point Of A Liquid Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. As the altitude increases, the boiling. In an open system this is called. What Raises The Boiling Point Of A Liquid.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting What Raises The Boiling Point Of A Liquid As the altitude increases, the boiling. In an open system this is called atmospheric pressure. Normally when we boil a liquid, we do so at atmospheric pressure. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Under this condition, addition of heat results in the transformation of the liquid into its. What Raises The Boiling Point Of A Liquid.
From facts.net
20 Fascinating Facts About Boiling Point Elevation What Raises The Boiling Point Of A Liquid A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. In an open system this is called atmospheric pressure. Under this condition, addition of heat results in the transformation of the liquid into its vapor without raising the temperature. The boiling point is the temperature at which the vapor. What Raises The Boiling Point Of A Liquid.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Raises The Boiling Point Of A Liquid Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. As the altitude increases, the boiling. Under this condition, addition of heat results in the transformation. What Raises The Boiling Point Of A Liquid.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Raises The Boiling Point Of A Liquid As the altitude increases, the boiling. As the altitude increases, the boiling point decreases. Under this condition, addition of heat results in the transformation of the liquid into its vapor without raising the temperature. The pressure of gas above a liquid affects the boiling point. Normally when we boil a liquid, we do so at atmospheric pressure. Boiling point, temperature. What Raises The Boiling Point Of A Liquid.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation, free download ID1624745 What Raises The Boiling Point Of A Liquid The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The pressure of gas above a liquid affects the boiling point. This is the boiling point which is usually quoted in chemical literature. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by. What Raises The Boiling Point Of A Liquid.
From www.youtube.com
Simplest Way To Understand Boiling Point & Vapor Pressure YouTube What Raises The Boiling Point Of A Liquid A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Under this condition, addition of heat results in the transformation of the liquid into its vapor without raising the temperature. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. If. What Raises The Boiling Point Of A Liquid.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Raises The Boiling Point Of A Liquid The pressure of gas above a liquid affects the boiling point. Normally when we boil a liquid, we do so at atmospheric pressure. As the altitude increases, the boiling point decreases. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The. What Raises The Boiling Point Of A Liquid.
From www.youtube.com
Determine the Boiling Point of a Given Organic Liquid, Chemistry What Raises The Boiling Point Of A Liquid The pressure of gas above a liquid affects the boiling point. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. As the altitude increases, the boiling point decreases. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. What Raises The Boiling Point Of A Liquid.
From www.youtube.com
Method To Determine Boiling Point Of A Liquid Basic Principles and What Raises The Boiling Point Of A Liquid In an open system this is called atmospheric pressure. As the altitude increases, the boiling point decreases. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The boiling. What Raises The Boiling Point Of A Liquid.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Raises The Boiling Point Of A Liquid The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. As the altitude increases, the boiling. The pressure of gas above a liquid affects the boiling point. Boiling point,. What Raises The Boiling Point Of A Liquid.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Raises The Boiling Point Of A Liquid The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. As the altitude increases, the boiling point decreases. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. In an open system this is called atmospheric pressure. If this pressure is the. What Raises The Boiling Point Of A Liquid.
From www.youtube.com
Binary Boiling Point Diagram of a LiquidLiquid Mixture YouTube What Raises The Boiling Point Of A Liquid This is the boiling point which is usually quoted in chemical literature. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. As the altitude increases, the boiling point decreases. As the. What Raises The Boiling Point Of A Liquid.
From www.slideserve.com
PPT MELTING AND BOILING PowerPoint Presentation, free download ID What Raises The Boiling Point Of A Liquid The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Under this condition, addition of heat results in the transformation of the liquid into its vapor without raising the temperature. Normally when we boil a liquid, we do so at atmospheric pressure. In an open system this is called atmospheric. What Raises The Boiling Point Of A Liquid.
From www.youtube.com
Measurement of boiling point of water practically YouTube What Raises The Boiling Point Of A Liquid A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. As the altitude increases, the boiling. The lower the pressure of a gas above a liquid, the lower the. What Raises The Boiling Point Of A Liquid.
From www.slideserve.com
PPT Chapter 13 continued… PowerPoint Presentation, free download ID What Raises The Boiling Point Of A Liquid The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred. What Raises The Boiling Point Of A Liquid.
From misael-blogzamora.blogspot.com
The Boiling Point of a Given Liquid Varies What Raises The Boiling Point Of A Liquid The pressure of gas above a liquid affects the boiling point. As the altitude increases, the boiling point decreases. In an open system this is called atmospheric pressure. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon. What Raises The Boiling Point Of A Liquid.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin What Raises The Boiling Point Of A Liquid Under this condition, addition of heat results in the transformation of the liquid into its vapor without raising the temperature. In an open system this is called atmospheric pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. As the altitude. What Raises The Boiling Point Of A Liquid.
From www.slideserve.com
PPT Properties and Changes in Matter PowerPoint Presentation, free What Raises The Boiling Point Of A Liquid The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. As the altitude increases, the boiling. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. As the altitude increases, the boiling point decreases. Boiling point, temperature at which the pressure exerted. What Raises The Boiling Point Of A Liquid.
From www.slideserve.com
PPT Chapter 10 States of Matter PowerPoint Presentation, free What Raises The Boiling Point Of A Liquid Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. A liquid boils at a temperature. What Raises The Boiling Point Of A Liquid.
From circuitwiringstroke123.z13.web.core.windows.net
Normal Boiling Point Chemistry What Raises The Boiling Point Of A Liquid The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. What Raises The Boiling Point Of A Liquid.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff What Raises The Boiling Point Of A Liquid The pressure of gas above a liquid affects the boiling point. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid; This is the boiling point which is usually quoted in chemical literature. If this pressure is the standard pressure of 1 atm (101.3. What Raises The Boiling Point Of A Liquid.
From www.youtube.com
CHEMISTRY 201 Calculating Boiling Point Using Clausius Clapeyron What Raises The Boiling Point Of A Liquid If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Under this condition, addition of heat results in the transformation of the liquid into its vapor without raising the temperature. In an open system this is called atmospheric pressure. Normally when we. What Raises The Boiling Point Of A Liquid.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox What Raises The Boiling Point Of A Liquid A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The lower the pressure of a gas above a liquid, the. What Raises The Boiling Point Of A Liquid.
From www.youtube.com
Boiling Points of Liquids, Chemistry Lecture Sabaq.pk YouTube What Raises The Boiling Point Of A Liquid The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Under this condition, addition of heat results in the transformation of the liquid into its vapor without raising the temperature. The pressure of gas above a liquid affects the boiling point. Normally when we boil a liquid, we do so. What Raises The Boiling Point Of A Liquid.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5694866 What Raises The Boiling Point Of A Liquid The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. As the altitude increases, the boiling point decreases. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid. What Raises The Boiling Point Of A Liquid.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 What Raises The Boiling Point Of A Liquid If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The pressure of gas above a liquid affects the boiling point.. What Raises The Boiling Point Of A Liquid.
From matmake.com
Boiling Point Definition, Measurements, and Applications What Raises The Boiling Point Of A Liquid Under this condition, addition of heat results in the transformation of the liquid into its vapor without raising the temperature. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Normally when we boil a liquid, we do so at atmospheric pressure. Boiling point, temperature at which the pressure exerted by the. What Raises The Boiling Point Of A Liquid.
From studylib.net
Boiling Point of Liquids What Raises The Boiling Point Of A Liquid Under this condition, addition of heat results in the transformation of the liquid into its vapor without raising the temperature. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Normally when we boil a liquid, we do so at atmospheric pressure. If this pressure is the standard pressure of 1 atm. What Raises The Boiling Point Of A Liquid.
From www.worksheetsplanet.com
What is Boiling Point What Raises The Boiling Point Of A Liquid If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. As the altitude increases, the boiling. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. As the altitude increases, the. What Raises The Boiling Point Of A Liquid.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Raises The Boiling Point Of A Liquid As the altitude increases, the boiling. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. This is the boiling point which is usually quoted in chemical literature. The boiling point is the temperature at which the vapor pressure of a liquid. What Raises The Boiling Point Of A Liquid.
From guidemanualeruptivity.z14.web.core.windows.net
Chemistry Boiling Point Chart What Raises The Boiling Point Of A Liquid As the altitude increases, the boiling. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Boiling point, temperature at which the. What Raises The Boiling Point Of A Liquid.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point What Raises The Boiling Point Of A Liquid The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Normally when we boil a liquid, we do so at atmospheric pressure.. What Raises The Boiling Point Of A Liquid.