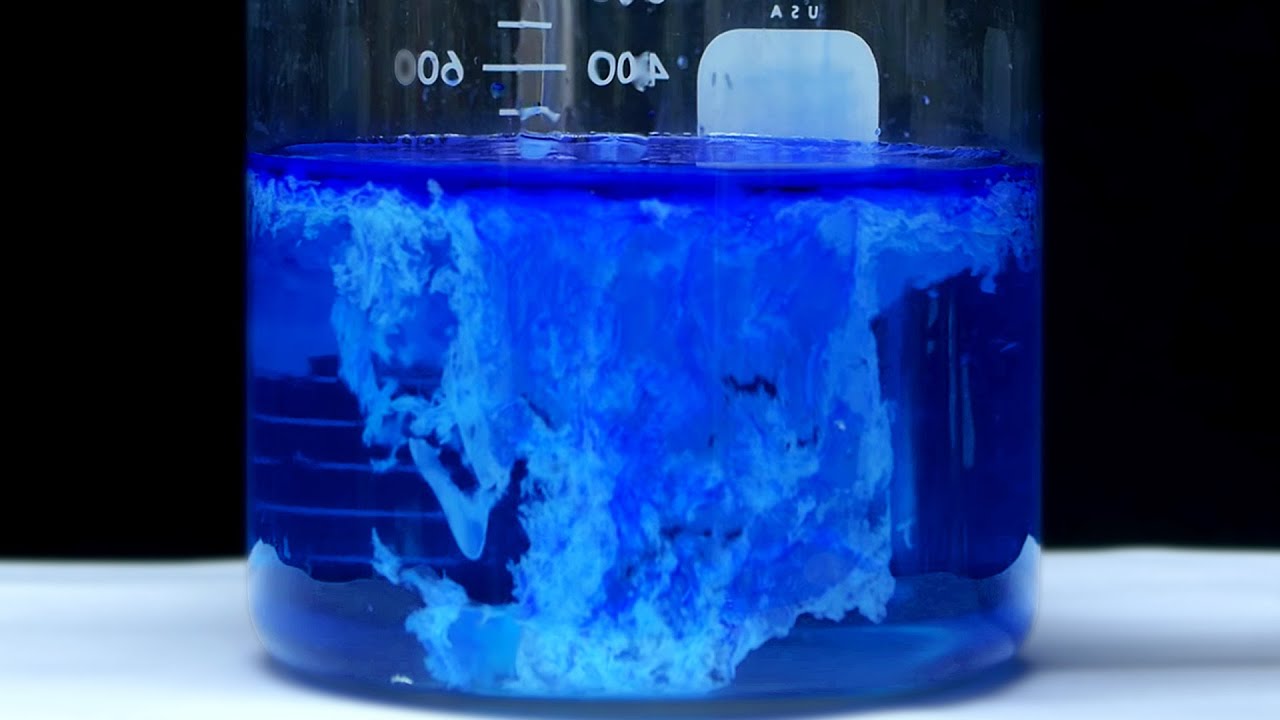
Copper 2 Hydroxide + Heat . As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. But isn't it a solid by itself too? The reaction taking place is. The decomposition equation for copper ii hydroxide is: Explore the unique compound copper (ii) hydroxide: What is the copper ii hydroxide decomposition equation? If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Its structure, properties, synthesis, applications, and environmental impact. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated.
from www.youtube.com
Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. The decomposition equation for copper ii hydroxide is: What is the copper ii hydroxide decomposition equation? Explore the unique compound copper (ii) hydroxide: If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. Its structure, properties, synthesis, applications, and environmental impact. But isn't it a solid by itself too? The reaction taking place is. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated.
Making copper hydroxide YouTube
Copper 2 Hydroxide + Heat What is the copper ii hydroxide decomposition equation? So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. The reaction taking place is. If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Explore the unique compound copper (ii) hydroxide: But isn't it a solid by itself too? What is the copper ii hydroxide decomposition equation? The decomposition equation for copper ii hydroxide is: As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. Its structure, properties, synthesis, applications, and environmental impact.
From www.youtube.com
How to write the formula for copper (II) hydroxide YouTube Copper 2 Hydroxide + Heat Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. The reaction taking place is. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. What is the copper ii hydroxide decomposition equation?. Copper 2 Hydroxide + Heat.
From www.youtube.com
Making copper hydroxide YouTube Copper 2 Hydroxide + Heat So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Explore the unique compound copper (ii) hydroxide: The decomposition equation for copper ii hydroxide is: Its structure, properties, synthesis, applications, and environmental impact. But isn't it a solid by itself too? What is the copper ii hydroxide decomposition equation? If you add concentrated hydrochloric acid to a solution containing. Copper 2 Hydroxide + Heat.
From woelen.homescience.net
Science made alive Chemistry/Solutions Copper 2 Hydroxide + Heat If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. But isn't it a solid by itself too? The reaction taking place is. As a result, hydroxide ion can displace water from the copper (ii) ion,. Copper 2 Hydroxide + Heat.
From www.youtube.com
of Copper(II) Carbonate Hydroxide Hydrate in LateNiteLabs Copper 2 Hydroxide + Heat But isn't it a solid by itself too? What is the copper ii hydroxide decomposition equation? So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. Its structure, properties, synthesis, applications, and environmental impact. The reaction taking. Copper 2 Hydroxide + Heat.
From www.youtube.com
How to Write the Net Ionic Equation for Cu(OH)2 = CuO + H2O YouTube Copper 2 Hydroxide + Heat The reaction taking place is. Its structure, properties, synthesis, applications, and environmental impact. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. But isn't it a solid by itself too? If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the. Copper 2 Hydroxide + Heat.
From www.youtube.com
How to find the percent composition of Cu(OH)2 (Copper (II) Hydroxide Copper 2 Hydroxide + Heat What is the copper ii hydroxide decomposition equation? The decomposition equation for copper ii hydroxide is: But isn't it a solid by itself too? The reaction taking place is. Its structure, properties, synthesis, applications, and environmental impact. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. As a result, hydroxide ion can displace water from the copper (ii). Copper 2 Hydroxide + Heat.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free Copper 2 Hydroxide + Heat Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. The reaction taking place is. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. Its structure, properties, synthesis, applications, and environmental. Copper 2 Hydroxide + Heat.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation Copper 2 Hydroxide + Heat Explore the unique compound copper (ii) hydroxide: What is the copper ii hydroxide decomposition equation? Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. The reaction taking place is. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. But isn't it a solid by itself too? The decomposition equation for copper ii hydroxide. Copper 2 Hydroxide + Heat.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Copper 2 Hydroxide + Heat What is the copper ii hydroxide decomposition equation? The decomposition equation for copper ii hydroxide is: As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. So just wondering, copper(ii) hydroxide undergoes thermal decomposition. Copper 2 Hydroxide + Heat.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper 2 Hydroxide + Heat As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Explore the unique compound copper (ii) hydroxide: The decomposition equation for copper ii hydroxide is: But isn't it a solid by itself too? The. Copper 2 Hydroxide + Heat.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper 2 Hydroxide + Heat So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Explore the unique compound copper (ii) hydroxide: Its structure, properties, synthesis, applications, and environmental impact. If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. The reaction taking place is. What is the copper ii hydroxide. Copper 2 Hydroxide + Heat.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Copper 2 Hydroxide + Heat The reaction taking place is. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. But isn't it a solid by itself too? Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. The decomposition equation for copper ii hydroxide is: Explore the unique. Copper 2 Hydroxide + Heat.
From woelen.homescience.net
Science made alive Chemistry/Solutions Copper 2 Hydroxide + Heat What is the copper ii hydroxide decomposition equation? If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. The reaction taking place is. But isn't it a solid by itself too? Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. The decomposition. Copper 2 Hydroxide + Heat.
From www.youtube.com
How to Balance Cu(OH)2= CuO + H2O YouTube Copper 2 Hydroxide + Heat What is the copper ii hydroxide decomposition equation? If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. Its structure, properties, synthesis, applications, and environmental. Copper 2 Hydroxide + Heat.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Copper 2 Hydroxide + Heat The decomposition equation for copper ii hydroxide is: So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Explore the unique compound copper (ii) hydroxide: Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a. Copper 2 Hydroxide + Heat.
From hxeddpfhv.blob.core.windows.net
Copper Hydroxide State at Madeline Sumner blog Copper 2 Hydroxide + Heat The reaction taking place is. What is the copper ii hydroxide decomposition equation? Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Its structure, properties, synthesis, applications, and environmental impact. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding. Copper 2 Hydroxide + Heat.
From www.online-sciences.com
Types of chemical reactions and Thermal reactions Copper 2 Hydroxide + Heat If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. The decomposition equation for copper ii hydroxide is: What is the copper ii hydroxide decomposition equation? Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. The reaction taking place is. Explore the. Copper 2 Hydroxide + Heat.
From www.youtube.com
Type of Reaction for Cu(OH)2 = CuO + H2O YouTube Copper 2 Hydroxide + Heat The reaction taking place is. Its structure, properties, synthesis, applications, and environmental impact. But isn't it a solid by itself too? If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. What is the copper ii hydroxide decomposition equation? The decomposition equation for copper ii hydroxide is:. Copper 2 Hydroxide + Heat.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Copper 2 Hydroxide + Heat As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. The decomposition equation for copper ii hydroxide is: Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. But isn't it a solid by itself too? So just wondering, copper(ii) hydroxide undergoes thermal decomposition. Copper 2 Hydroxide + Heat.
From www.youtube.com
Copper in Copper I hydroxide and Copper II hydroxide YouTube Copper 2 Hydroxide + Heat So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Explore the unique compound copper (ii) hydroxide: The reaction taking place is. What is the copper ii hydroxide decomposition equation? As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. Its structure, properties, synthesis, applications, and environmental. Copper 2 Hydroxide + Heat.
From www.youtube.com
How to Write the Formula for Copper (II) hydroxide YouTube Copper 2 Hydroxide + Heat If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. The decomposition equation for copper ii hydroxide is: So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. But isn't it a solid by itself too? What is the copper ii hydroxide decomposition equation? Copper(ii) ion. Copper 2 Hydroxide + Heat.
From www.youtube.com
How to Write the Equation for Cu(OH)2 + H2O YouTube Copper 2 Hydroxide + Heat Its structure, properties, synthesis, applications, and environmental impact. Explore the unique compound copper (ii) hydroxide: So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. What is the copper ii hydroxide decomposition equation? But isn't it a solid by itself too? As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh). Copper 2 Hydroxide + Heat.
From www.youtube.com
Make Copper II Oxide, and Copper II Hydroxide YouTube Copper 2 Hydroxide + Heat The decomposition equation for copper ii hydroxide is: But isn't it a solid by itself too? If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. Explore the unique compound copper (ii) hydroxide: Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2.. Copper 2 Hydroxide + Heat.
From www.numerade.com
SOLVED 2 REACTION I Dissolution of copper metal with nitric acid Copper 2 Hydroxide + Heat So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Explore the unique compound copper (ii) hydroxide: But isn't it a solid by itself too? Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a. Copper 2 Hydroxide + Heat.
From bnrc.springeropen.com
Copper(II) oxide nanocatalyst preparation and characterization green Copper 2 Hydroxide + Heat Its structure, properties, synthesis, applications, and environmental impact. What is the copper ii hydroxide decomposition equation? So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. The reaction taking place is. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. If you add concentrated hydrochloric acid. Copper 2 Hydroxide + Heat.
From www.ceramic-glazes.com
Copper Hydroxide Cupric hydroxide patina for ceramics Copper 2 Hydroxide + Heat But isn't it a solid by itself too? So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. What is the copper ii hydroxide decomposition equation? If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. The reaction taking place is. Explore the unique compound copper. Copper 2 Hydroxide + Heat.
From www.youtube.com
What kind of reaction is Copper(II) nitrate (Cu(NO3)2) and Sodium Copper 2 Hydroxide + Heat So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. The decomposition equation for copper ii hydroxide is: As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. The reaction taking place is. But isn't it a solid by itself too? What is the copper ii hydroxide. Copper 2 Hydroxide + Heat.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper 2 Hydroxide + Heat But isn't it a solid by itself too? If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. Explore the unique compound copper (ii) hydroxide: The decomposition equation for copper ii hydroxide is: Its structure, properties, synthesis, applications, and environmental impact. Copper(ii) ion reacts with stoichiometric quantities. Copper 2 Hydroxide + Heat.
From testbook.com
Copper Hydroxide Learn Definition, Structure, Formula, Uses here Copper 2 Hydroxide + Heat Its structure, properties, synthesis, applications, and environmental impact. Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. What is the copper ii hydroxide decomposition equation? The reaction taking place is. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Explore the unique compound copper (ii) hydroxide: If you add concentrated hydrochloric acid to. Copper 2 Hydroxide + Heat.
From www.slideserve.com
PPT SURVEY OF CHEMISTRY LABORATORY I CHEM 1151L REACTIONS OF COPPER Copper 2 Hydroxide + Heat What is the copper ii hydroxide decomposition equation? Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. The decomposition equation for copper ii hydroxide is: Its structure, properties, synthesis, applications, and environmental impact. Explore the unique compound copper (ii) hydroxide: But isn't it a solid by itself too? As a result, hydroxide ion can displace. Copper 2 Hydroxide + Heat.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Copper 2 Hydroxide + Heat Explore the unique compound copper (ii) hydroxide: The reaction taking place is. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. Its structure, properties, synthesis, applications, and environmental impact. If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. What is the copper ii hydroxide. Copper 2 Hydroxide + Heat.
From www.youtube.com
4_Copper(II) hydroxide + Heat YouTube Copper 2 Hydroxide + Heat If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. The decomposition equation for copper ii hydroxide is: Explore the unique compound copper (ii) hydroxide:. Copper 2 Hydroxide + Heat.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation Copper 2 Hydroxide + Heat If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. But isn't it a. Copper 2 Hydroxide + Heat.
From www.shutterstock.com
Copperii Hydroxide Formula Cuoh2 Cuh2o2 Pale Stock Illustration Copper 2 Hydroxide + Heat As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. So just wondering, copper(ii) hydroxide undergoes thermal decomposition when it's heated. The decomposition equation for copper ii hydroxide is: Copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. But isn't it a solid. Copper 2 Hydroxide + Heat.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper 2 Hydroxide + Heat The decomposition equation for copper ii hydroxide is: As a result, hydroxide ion can displace water from the copper (ii) ion, yielding copper hydroxide, cu (oh) 2, a blue precipitate. The reaction taking place is. If you add concentrated hydrochloric acid to a solution containing hexaaquacopper (ii) ions, the six water molecules are replaced by four chloride ions. Its structure,. Copper 2 Hydroxide + Heat.