Internal Energy Of Water . Entropy s = 9.103679 j/g*k at 26.9 c and 0.0010 mpa. The energy required by different. Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. Changes in a material's temperature or state of matter are caused by changes to the internal energy. The first law of thermodynamics states that the energy of the universe is. Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. For an ideal gas, the case is simple. How does one calculate the total internal energy of a liter of water? $e = c*n*t$, where c is the. Thermodynamic property calculator for water: A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,.
from www.chegg.com
$e = c*n*t$, where c is the. Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. The first law of thermodynamics states that the energy of the universe is. Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. The energy required by different. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. For an ideal gas, the case is simple. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,. Entropy s = 9.103679 j/g*k at 26.9 c and 0.0010 mpa. Changes in a material's temperature or state of matter are caused by changes to the internal energy.
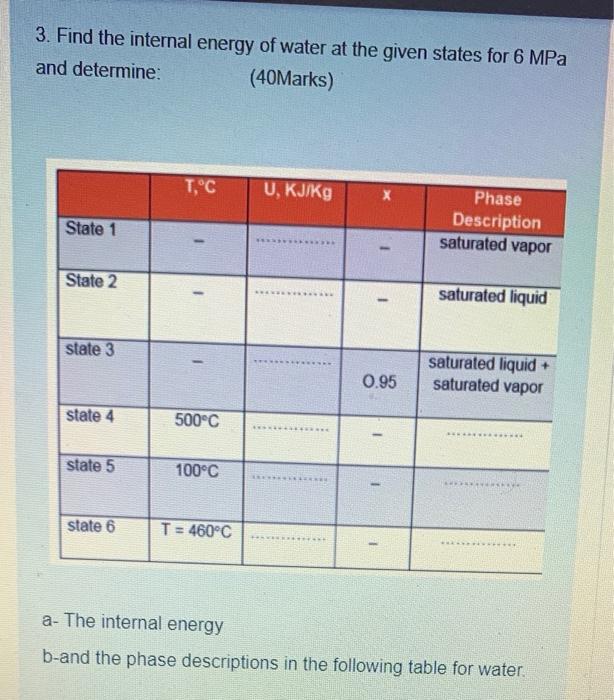
3. Find the internal energy of water at the given
Internal Energy Of Water A reaction or process in which heat is transferred to a system from its surroundings is endothermic. How does one calculate the total internal energy of a liter of water? For an ideal gas, the case is simple. $e = c*n*t$, where c is the. The first law of thermodynamics states that the energy of the universe is. The energy required by different. Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,. Changes in a material's temperature or state of matter are caused by changes to the internal energy. Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. Entropy s = 9.103679 j/g*k at 26.9 c and 0.0010 mpa. Thermodynamic property calculator for water: A reaction or process in which heat is transferred to a system from its surroundings is endothermic.
From www.chegg.com
3. Find the internal energy of water at the given Internal Energy Of Water Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. $e = c*n*t$, where c is the. How does one calculate the total internal energy of a liter of water? Changes in a material's temperature or state of matter are caused by changes to the internal energy. A reaction or process in which heat is transferred to a system. Internal Energy Of Water.
From www.energyfrontier.us
The World of Water Science Energy Frontier Research Center Internal Energy Of Water Changes in a material's temperature or state of matter are caused by changes to the internal energy. Thermodynamic property calculator for water: The first law of thermodynamics states that the energy of the universe is. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,. For an ideal gas, the case is. Internal Energy Of Water.
From www.chegg.com
Solved Problem 03.032 Specific internal energy of water Internal Energy Of Water Thermodynamic property calculator for water: Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. For an ideal gas, the case is simple. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,. $e = c*n*t$, where c is the. Changes in a material's temperature or state of matter are caused. Internal Energy Of Water.
From www.chegg.com
Solved Find the specific internal energy of water after the Internal Energy Of Water The energy required by different. For an ideal gas, the case is simple. The first law of thermodynamics states that the energy of the universe is. Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. How does one calculate the total internal energy of a liter of water? $e = c*n*t$, where c is the. Density, heat capacity. Internal Energy Of Water.
From www.chegg.com
Solved Find the internal energy of water at the given states Internal Energy Of Water Changes in a material's temperature or state of matter are caused by changes to the internal energy. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,. $e = c*n*t$, where c is the. The energy required by different. For an ideal gas, the case is simple. How does one calculate the. Internal Energy Of Water.
From studylib.net
INTERNAL ENERGY OF WATER MOLECULE Internal Energy Of Water The first law of thermodynamics states that the energy of the universe is. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. $e = c*n*t$, where c is the. Changes. Internal Energy Of Water.
From www.coursehero.com
[Solved] 3. Find the internal energy of water at the given states for 6 Internal Energy Of Water For an ideal gas, the case is simple. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,. Changes in a material's temperature or state of matter are caused by changes to the internal energy. Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. A reaction or process in which. Internal Energy Of Water.
From www.vanderbilt.edu
Importance of Water SustainVU Vanderbilt University Internal Energy Of Water $e = c*n*t$, where c is the. Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. The first law of thermodynamics states that the energy of the universe is. Changes in a material's temperature or state of matter are caused by changes to the internal energy. Entropy s = 9.103679 j/g*k at 26.9 c and 0.0010 mpa. How. Internal Energy Of Water.
From slidetodoc.com
PTT 2014 THERMODYNAMIC SEM 1 20132014 CHAPTER 3 Internal Energy Of Water Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. The first law of thermodynamics states that the energy of the universe is. How does one calculate the total internal energy of a liter of water? Entropy s = 9.103679 j/g*k at 26.9 c and 0.0010 mpa. Figures and tables showing how the properties of water changes along the. Internal Energy Of Water.
From pressbooks.bccampus.ca
4.1 Internal energy in a system Introduction to Engineering Internal Energy Of Water Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. For an ideal gas, the case is simple. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,. Changes in a material's temperature or state of matter are caused by changes to the internal energy. A reaction or process in which. Internal Energy Of Water.
From sharkresearch.earth.miami.edu
Powering the World with the Energy of Water Shark Research Internal Energy Of Water $e = c*n*t$, where c is the. Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. The first law of thermodynamics states that the energy of the universe is. How does one calculate the total internal energy of a liter of water? Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. Changes in a material's temperature. Internal Energy Of Water.
From www.slideserve.com
PPT ENGSC 2333 Thermodynamics Chapter 3 PowerPoint Presentation Internal Energy Of Water Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. For an ideal gas, the case is simple. $e = c*n*t$, where c is the. Thermodynamic property calculator for water: Entropy s = 9.103679 j/g*k at 26.9 c and 0.0010 mpa. The energy required by different. Changes in a material's temperature or state of matter are caused by changes. Internal Energy Of Water.
From www.slideserve.com
PPT CE 374K Hydrology PowerPoint Presentation ID684554 Internal Energy Of Water How does one calculate the total internal energy of a liter of water? Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. Thermodynamic property calculator for water: The first law of thermodynamics states that the energy of the universe is. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,.. Internal Energy Of Water.
From www.chegg.com
Solved Determine the internal energy (in kJ/kg) of Internal Energy Of Water Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. Entropy s = 9.103679 j/g*k at 26.9 c and 0.0010 mpa. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. For an ideal gas, the case is simple. The first law of thermodynamics states that the energy of the universe is.. Internal Energy Of Water.
From www.numerade.com
1. Find the internal energy of water at the given states for 7 MPa and Internal Energy Of Water Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. Changes in a material's temperature or state of matter are caused by changes to the internal energy. Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. How does one calculate the total internal energy of a liter of water? For an ideal gas, the case is simple.. Internal Energy Of Water.
From www.chegg.com
Solved Find the internal energy of water at the given states Internal Energy Of Water Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. $e = c*n*t$, where c is the. For an ideal gas, the case is simple. Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. Thermodynamic property calculator for water: Entropy s = 9.103679 j/g*k at 26.9 c and 0.0010 mpa. How does one calculate the total internal. Internal Energy Of Water.
From www.grc.nasa.gov
Enthalpy Internal Energy Of Water Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,. $e = c*n*t$, where c is the. Thermodynamic property calculator for water: For an ideal gas, the case is simple. The energy required by different. Changes in a material's temperature or state of matter are caused by changes to the internal energy.. Internal Energy Of Water.
From www.tec-science.com
Internal energy & first law of thermodynamics tecscience Internal Energy Of Water The first law of thermodynamics states that the energy of the universe is. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. Thermodynamic property calculator for water: Changes in a material's temperature or state of matter are caused by changes to. Internal Energy Of Water.
From www.numerade.com
SOLVED Identify how heat causes the increase of internal energy of Internal Energy Of Water $e = c*n*t$, where c is the. Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. Thermodynamic property calculator for water: A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Figures and tables showing how the properties of water. Internal Energy Of Water.
From www.tec-science.com
Internal energy & first law of thermodynamics tecscience Internal Energy Of Water A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The energy required by different. Changes in a material's temperature or state of matter are caused by changes to the internal energy. Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. How does one calculate the total internal energy of a. Internal Energy Of Water.
From www.researchgate.net
Internal energy of water versus temperature. A transition point (a Internal Energy Of Water $e = c*n*t$, where c is the. Changes in a material's temperature or state of matter are caused by changes to the internal energy. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The energy required by different. Figures and tables showing how the properties of water changes along the boiling/condensation curve. Internal Energy Of Water.
From www.youtube.com
Assuming the water vapor to be a perfect gas, calculate the internal Internal Energy Of Water The first law of thermodynamics states that the energy of the universe is. For an ideal gas, the case is simple. Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. Changes in a material's temperature or state of matter are caused by changes to the internal energy. A reaction or process in which heat is transferred to a. Internal Energy Of Water.
From www.chegg.com
Solved Thermodynamics Specific internal energy of water at Internal Energy Of Water Changes in a material's temperature or state of matter are caused by changes to the internal energy. $e = c*n*t$, where c is the. The energy required by different. Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,. Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. How does. Internal Energy Of Water.
From study.com
How to Find the Internal Energy of a System Chemistry Internal Energy Of Water The first law of thermodynamics states that the energy of the universe is. $e = c*n*t$, where c is the. Thermodynamic property calculator for water: How does one calculate the total internal energy of a liter of water? For an ideal gas, the case is simple. Entropy s = 9.103679 j/g*k at 26.9 c and 0.0010 mpa. Changes in a. Internal Energy Of Water.
From www.iso.org
ISO The Power of Water Internal Energy Of Water $e = c*n*t$, where c is the. Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Changes in a material's temperature or state of matter are caused by changes to the internal energy. Entropy s = 9.103679 j/g*k at 26.9 c. Internal Energy Of Water.
From www.chegg.com
Solved What is the internal energy of water at 500 psia and Internal Energy Of Water Entropy s = 9.103679 j/g*k at 26.9 c and 0.0010 mpa. How does one calculate the total internal energy of a liter of water? The first law of thermodynamics states that the energy of the universe is. Changes in a material's temperature or state of matter are caused by changes to the internal energy. For an ideal gas, the case. Internal Energy Of Water.
From www.chegg.com
Solved Find the internal energy of water at the following Internal Energy Of Water Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,. For an ideal gas, the case is simple. Changes in a material's temperature or state of matter are caused by changes to the internal energy. Entropy s = 9.103679 j/g*k at 26.9 c and 0.0010 mpa. The energy required by different. $e. Internal Energy Of Water.
From www.researchgate.net
(a) The internal energy, and (b) pressure, of the short range TIP4P Internal Energy Of Water Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,. Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. Thermodynamic property calculator for water: A reaction or process in which heat is transferred to a system from its surroundings is endothermic. For an ideal gas, the case is simple. Entropy. Internal Energy Of Water.
From www.chegg.com
Solved Find the internal energy of water at the given states Internal Energy Of Water How does one calculate the total internal energy of a liter of water? $e = c*n*t$, where c is the. Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. The first law of thermodynamics states that the energy of the universe is. Changes in a material's temperature or state of matter are caused by changes to the internal. Internal Energy Of Water.
From www.youtube.com
Thermodynamics 332 What is the specific internal energy of water at 50 Internal Energy Of Water Changes in a material's temperature or state of matter are caused by changes to the internal energy. $e = c*n*t$, where c is the. Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. The energy required by different. For an ideal gas, the case is simple. How does one calculate the total internal energy of a liter of. Internal Energy Of Water.
From www.chegg.com
Solved Question 2 (10 points)The internal energy of water Internal Energy Of Water $e = c*n*t$, where c is the. Thermodynamic property calculator for water: A reaction or process in which heat is transferred to a system from its surroundings is endothermic. The energy required by different. Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. How does one calculate the total internal energy of a liter of water? Changes in. Internal Energy Of Water.
From www.researchgate.net
Internal energy of water and vapour as function of the temperature Internal Energy Of Water Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. Entropy s = 9.103679 j/g*k at 26.9 c and 0.0010 mpa. The energy required by different. Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity,. Internal Energy Of Water.
From www.numerade.com
SOLVED Determine the internal energy of water at 20 psia and 400°F. Internal Energy Of Water Changes in a material's temperature or state of matter are caused by changes to the internal energy. Thermodynamic property calculator for water: Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. $e = c*n*t$, where c is the. For an ideal gas, the case is simple. The first law of thermodynamics states that the energy of the universe. Internal Energy Of Water.
From www.coursehero.com
[Solved] 3. Find the internal energy of water at the given states for 6 Internal Energy Of Water Density, heat capacity (isobaric and isochoric), enthalpy, entropy, conductivity, viscosity etc. Thermodynamic property calculator for water: Enthalpy h = 2551.013479 kj/kg at 26.9 c and 0.0010 mpa. A reaction or process in which heat is transferred to a system from its surroundings is endothermic. How does one calculate the total internal energy of a liter of water? For an ideal. Internal Energy Of Water.
From hxerdjgqx.blob.core.windows.net
Internal Energy Water at Howard blog Internal Energy Of Water For an ideal gas, the case is simple. The energy required by different. Changes in a material's temperature or state of matter are caused by changes to the internal energy. Thermodynamic property calculator for water: Figures and tables showing how the properties of water changes along the boiling/condensation curve (vapor pressure, density, viscosity,. $e = c*n*t$, where c is the.. Internal Energy Of Water.