Titration With An Acid And A Base Lab Answers . Titration is an analytical quantitative technique used to determine the concentration of a solute; To use a previously standardized solution to determine the concentration of an unknown acid. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In equation 1, the acid is hcl. Study with quizlet and memorize flashcards. Precise density of your standardized solution of naoh. Calculations b for determination of molecular weight of an unknown acid. By observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration curves. The analyte (titrand) is the solution with an. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or.
from www.transtutors.com
The analyte (titrand) is the solution with an. In equation 1, the acid is hcl. To use a previously standardized solution to determine the concentration of an unknown acid. Calculations b for determination of molecular weight of an unknown acid. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Study with quizlet and memorize flashcards. Titration is an analytical quantitative technique used to determine the concentration of a solute; Precise density of your standardized solution of naoh. By observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration curves.
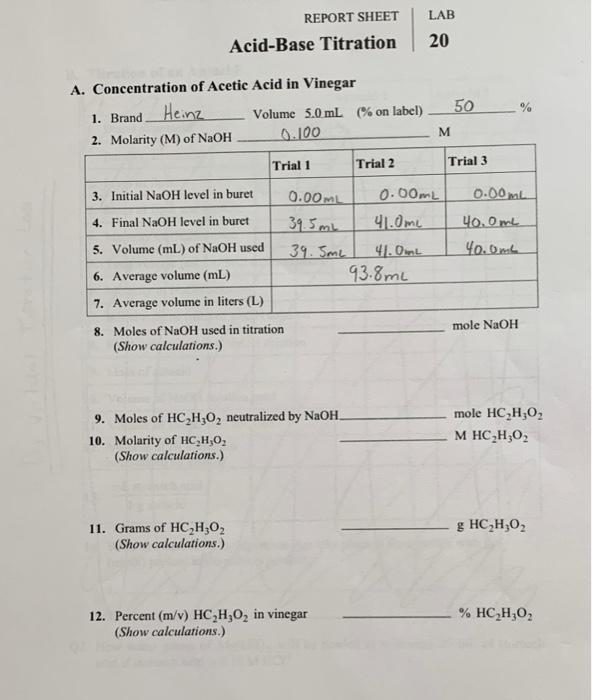
(Get Answer) LAB REPORT SHEET AcidBase Titration 20 50 A
Titration With An Acid And A Base Lab Answers The analyte (titrand) is the solution with an. The analyte (titrand) is the solution with an. In equation 1, the acid is hcl. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Study with quizlet and memorize flashcards. By observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration curves. Titration is an analytical quantitative technique used to determine the concentration of a solute; To use a previously standardized solution to determine the concentration of an unknown acid. Precise density of your standardized solution of naoh. Calculations b for determination of molecular weight of an unknown acid.
From www.vrogue.co
Acid Base Titration Lab Questions Answers vrogue.co Titration With An Acid And A Base Lab Answers By observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration curves. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: To use a previously standardized solution to determine the concentration. Titration With An Acid And A Base Lab Answers.
From www.numerade.com
SOLVED Text EXPERIMENT 6 ACIDBASE TITRATIONS LABORATORY REPORT SHEET Titration With An Acid And A Base Lab Answers Titration is an analytical quantitative technique used to determine the concentration of a solute; In equation 1, the acid is hcl. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: The analyte (titrand) is the solution with an. Precise density of your standardized solution of naoh. Study. Titration With An Acid And A Base Lab Answers.
From www.docsity.com
Acid base titration lab answers Docsity Titration With An Acid And A Base Lab Answers To use a previously standardized solution to determine the concentration of an unknown acid. Titration is an analytical quantitative technique used to determine the concentration of a solute; One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Study with quizlet and memorize flashcards. The analyte (titrand) is. Titration With An Acid And A Base Lab Answers.
From www.chegg.com
REPORT SHEET LAB AcidBase Titration 12 A. Concent... Titration With An Acid And A Base Lab Answers To use a previously standardized solution to determine the concentration of an unknown acid. Titration is an analytical quantitative technique used to determine the concentration of a solute; By observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration curves. The analyte (titrand) is. Titration With An Acid And A Base Lab Answers.
From soetrust.org
2022 UPDATED!!! Acid base titration lab answers Soetrust Titration With An Acid And A Base Lab Answers Calculations b for determination of molecular weight of an unknown acid. Study with quizlet and memorize flashcards. Titration is an analytical quantitative technique used to determine the concentration of a solute; Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. To use a previously standardized solution to. Titration With An Acid And A Base Lab Answers.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration With An Acid And A Base Lab Answers Study with quizlet and memorize flashcards. Precise density of your standardized solution of naoh. The analyte (titrand) is the solution with an. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration is an analytical quantitative technique used to determine the concentration of a solute; By observing. Titration With An Acid And A Base Lab Answers.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Titration With An Acid And A Base Lab Answers The analyte (titrand) is the solution with an. To use a previously standardized solution to determine the concentration of an unknown acid. In equation 1, the acid is hcl. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Study with quizlet and memorize flashcards. Precise density of. Titration With An Acid And A Base Lab Answers.
From www.chegg.com
Solved Experiment 17B AcidBase Titration OBJECTIVES In Titration With An Acid And A Base Lab Answers Study with quizlet and memorize flashcards. To use a previously standardized solution to determine the concentration of an unknown acid. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration is an analytical quantitative technique used to determine the concentration of a solute; The analyte (titrand) is. Titration With An Acid And A Base Lab Answers.
From education2research.com
Understanding Acid Base Titration Pre Lab Questions and Answers Unraveled Titration With An Acid And A Base Lab Answers Titration is an analytical quantitative technique used to determine the concentration of a solute; Calculations b for determination of molecular weight of an unknown acid. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. To use a previously standardized solution to determine the concentration of an unknown. Titration With An Acid And A Base Lab Answers.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration With An Acid And A Base Lab Answers Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Titration is an analytical quantitative technique used to determine the concentration of a solute; To use. Titration With An Acid And A Base Lab Answers.
From studyfinder.org
Unlocking the Mysteries Titration of Acids and Bases Lab Answers Revealed Titration With An Acid And A Base Lab Answers Calculations b for determination of molecular weight of an unknown acid. To use a previously standardized solution to determine the concentration of an unknown acid. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Study with quizlet and memorize flashcards. By observing the titration of a strong. Titration With An Acid And A Base Lab Answers.
From www.transtutors.com
(Get Answer) LAB REPORT SHEET AcidBase Titration 20 50 A Titration With An Acid And A Base Lab Answers The analyte (titrand) is the solution with an. To use a previously standardized solution to determine the concentration of an unknown acid. In equation 1, the acid is hcl. Calculations b for determination of molecular weight of an unknown acid. Precise density of your standardized solution of naoh. By observing the titration of a strong acid and strong base and. Titration With An Acid And A Base Lab Answers.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Titration With An Acid And A Base Lab Answers One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: To use a previously standardized solution to determine the concentration of an unknown acid. In equation 1, the acid is hcl. Be able to determine the k a or k b from ph data associated with the titration. Titration With An Acid And A Base Lab Answers.
From www.chegg.com
Solved REPORT EXPERIMENT 9 ACID BASE TITRATION REPORT Titration With An Acid And A Base Lab Answers Titration is an analytical quantitative technique used to determine the concentration of a solute; Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. By observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes. Titration With An Acid And A Base Lab Answers.
From melanie-chapter.blogspot.com
Titration Of Acids And Bases Lab Report Experiment 20 Answers 45+ Pages Titration With An Acid And A Base Lab Answers The analyte (titrand) is the solution with an. In equation 1, the acid is hcl. Study with quizlet and memorize flashcards. Precise density of your standardized solution of naoh. Titration is an analytical quantitative technique used to determine the concentration of a solute; Calculations b for determination of molecular weight of an unknown acid. To use a previously standardized solution. Titration With An Acid And A Base Lab Answers.
From mavink.com
Acid Base Titration Lab Procedure Titration With An Acid And A Base Lab Answers In equation 1, the acid is hcl. The analyte (titrand) is the solution with an. Calculations b for determination of molecular weight of an unknown acid. Study with quizlet and memorize flashcards. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. One type of titration uses a. Titration With An Acid And A Base Lab Answers.
From www.chegg.com
Solved REPORT SHEET AcidBase Titration LAB 20 1. Brand Titration With An Acid And A Base Lab Answers Study with quizlet and memorize flashcards. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. To use a previously standardized solution to determine the concentration of an unknown acid. One type of titration uses a neutralization reaction, in which an acid and a base react to produce. Titration With An Acid And A Base Lab Answers.
From avery-yersbloglester.blogspot.com
Acid Base Titration Lab Report Answers Titration With An Acid And A Base Lab Answers The analyte (titrand) is the solution with an. Precise density of your standardized solution of naoh. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Calculations b for determination of molecular weight of an unknown acid. In equation 1, the acid is hcl. One type of titration. Titration With An Acid And A Base Lab Answers.
From mungfali.com
Acid Base Titration Lab Titration With An Acid And A Base Lab Answers Precise density of your standardized solution of naoh. Titration is an analytical quantitative technique used to determine the concentration of a solute; The analyte (titrand) is the solution with an. By observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration curves. Be able. Titration With An Acid And A Base Lab Answers.
From studyfinder.org
Unveiling Acid Base Titration Pre Lab Answers A Comprehensive Guide Titration With An Acid And A Base Lab Answers In equation 1, the acid is hcl. Calculations b for determination of molecular weight of an unknown acid. To use a previously standardized solution to determine the concentration of an unknown acid. Precise density of your standardized solution of naoh. By observing the titration of a strong acid and strong base and a strong base and weak acid one can. Titration With An Acid And A Base Lab Answers.
From melanie-chapter.blogspot.com
Titration Of Acids And Bases Lab Report Experiment 20 Answers 45+ Pages Titration With An Acid And A Base Lab Answers One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Study with quizlet and memorize flashcards. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. By observing the titration of a strong acid and strong. Titration With An Acid And A Base Lab Answers.
From www.chegg.com
Solved Experiment Acid/Base Titration Report Sheet Part 1 Titration With An Acid And A Base Lab Answers To use a previously standardized solution to determine the concentration of an unknown acid. In equation 1, the acid is hcl. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Precise density of your standardized solution of naoh. Titration is an analytical quantitative technique used to determine. Titration With An Acid And A Base Lab Answers.
From mungfali.com
Acid Base Titration Lab Titration With An Acid And A Base Lab Answers In equation 1, the acid is hcl. Calculations b for determination of molecular weight of an unknown acid. By observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration curves. Titration is an analytical quantitative technique used to determine the concentration of a solute;. Titration With An Acid And A Base Lab Answers.
From mavink.com
Acid Base Titration Lab Procedure Titration With An Acid And A Base Lab Answers Titration is an analytical quantitative technique used to determine the concentration of a solute; Study with quizlet and memorize flashcards. The analyte (titrand) is the solution with an. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. One type of titration uses a neutralization reaction, in which. Titration With An Acid And A Base Lab Answers.
From mungfali.com
Acid Base Titration Lab Titration With An Acid And A Base Lab Answers Precise density of your standardized solution of naoh. The analyte (titrand) is the solution with an. Calculations b for determination of molecular weight of an unknown acid. By observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration curves. In equation 1, the acid. Titration With An Acid And A Base Lab Answers.
From www.vrogue.co
Acid Base Titration Lab Answers vrogue.co Titration With An Acid And A Base Lab Answers Precise density of your standardized solution of naoh. Titration is an analytical quantitative technique used to determine the concentration of a solute; One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Study with quizlet and memorize flashcards. Be able to determine the k a or k b. Titration With An Acid And A Base Lab Answers.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 10 A. Titration With An Acid And A Base Lab Answers Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Calculations b for determination of molecular weight of an unknown acid. The analyte (titrand) is the solution with an. Titration is an analytical quantitative technique used to determine the concentration of a solute; One type of titration uses. Titration With An Acid And A Base Lab Answers.
From www.studypool.com
SOLUTION Acid Base Titration Datasheet Studypool Titration With An Acid And A Base Lab Answers By observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration curves. Calculations b for determination of molecular weight of an unknown acid. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and. Titration With An Acid And A Base Lab Answers.
From mungfali.com
Acid Base Titration Lab Titration With An Acid And A Base Lab Answers By observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration curves. The analyte (titrand) is the solution with an. To use a previously standardized solution to determine the concentration of an unknown acid. Study with quizlet and memorize flashcards. One type of titration. Titration With An Acid And A Base Lab Answers.
From moniquearesmeza.blogspot.com
Acid Base Titration Lab Report Answers MoniquearesMeza Titration With An Acid And A Base Lab Answers Calculations b for determination of molecular weight of an unknown acid. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. By observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration. Titration With An Acid And A Base Lab Answers.
From www.studypool.com
SOLUTION CHM 113 Experiment 8 Acid Base Titration Lab Report Studypool Titration With An Acid And A Base Lab Answers Study with quizlet and memorize flashcards. By observing the titration of a strong acid and strong base and a strong base and weak acid one can see how the shapes in the titration curves. Titration is an analytical quantitative technique used to determine the concentration of a solute; Calculations b for determination of molecular weight of an unknown acid. One. Titration With An Acid And A Base Lab Answers.
From studyfinder.org
Unlocking the Mysteries Titration of Acids and Bases Lab Answers Revealed Titration With An Acid And A Base Lab Answers To use a previously standardized solution to determine the concentration of an unknown acid. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Study with. Titration With An Acid And A Base Lab Answers.
From www.chegg.com
Solved REPORT SHEET Experiment 11 AcidBase Titration Titration With An Acid And A Base Lab Answers In equation 1, the acid is hcl. The analyte (titrand) is the solution with an. Study with quizlet and memorize flashcards. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. To use a previously standardized solution to determine the concentration of an unknown acid. One type of. Titration With An Acid And A Base Lab Answers.
From vasastephaniemackay.blogspot.com
Acid Base Titration Lab Report Answers Stephanie Mackay Titration With An Acid And A Base Lab Answers Calculations b for determination of molecular weight of an unknown acid. In equation 1, the acid is hcl. To use a previously standardized solution to determine the concentration of an unknown acid. Titration is an analytical quantitative technique used to determine the concentration of a solute; One type of titration uses a neutralization reaction, in which an acid and a. Titration With An Acid And A Base Lab Answers.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 12 . Titration With An Acid And A Base Lab Answers One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Precise density of your standardized solution of naoh. Study with quizlet and memorize flashcards. To use. Titration With An Acid And A Base Lab Answers.