How To Calculate Concentration From A Titration Curve . By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. \ce{naoh}\) is required to reach the end point when titrated. The volume of the titrant added. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Example \ (\pageindex {3}\) : Suppose that a titration is performed and \(20.70 \: Titration calculations are used to find the concentration of unknown solutions. They can also be used to calculate the ph after a given point during a titration. A titration is a volumetric technique in which a solution of one.
from psu.pb.unizin.org
They can also be used to calculate the ph after a given point during a titration. By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. Example \ (\pageindex {3}\) : Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Titration calculations are used to find the concentration of unknown solutions. \ce{naoh}\) is required to reach the end point when titrated. A titration is a volumetric technique in which a solution of one. The volume of the titrant added. Suppose that a titration is performed and \(20.70 \:
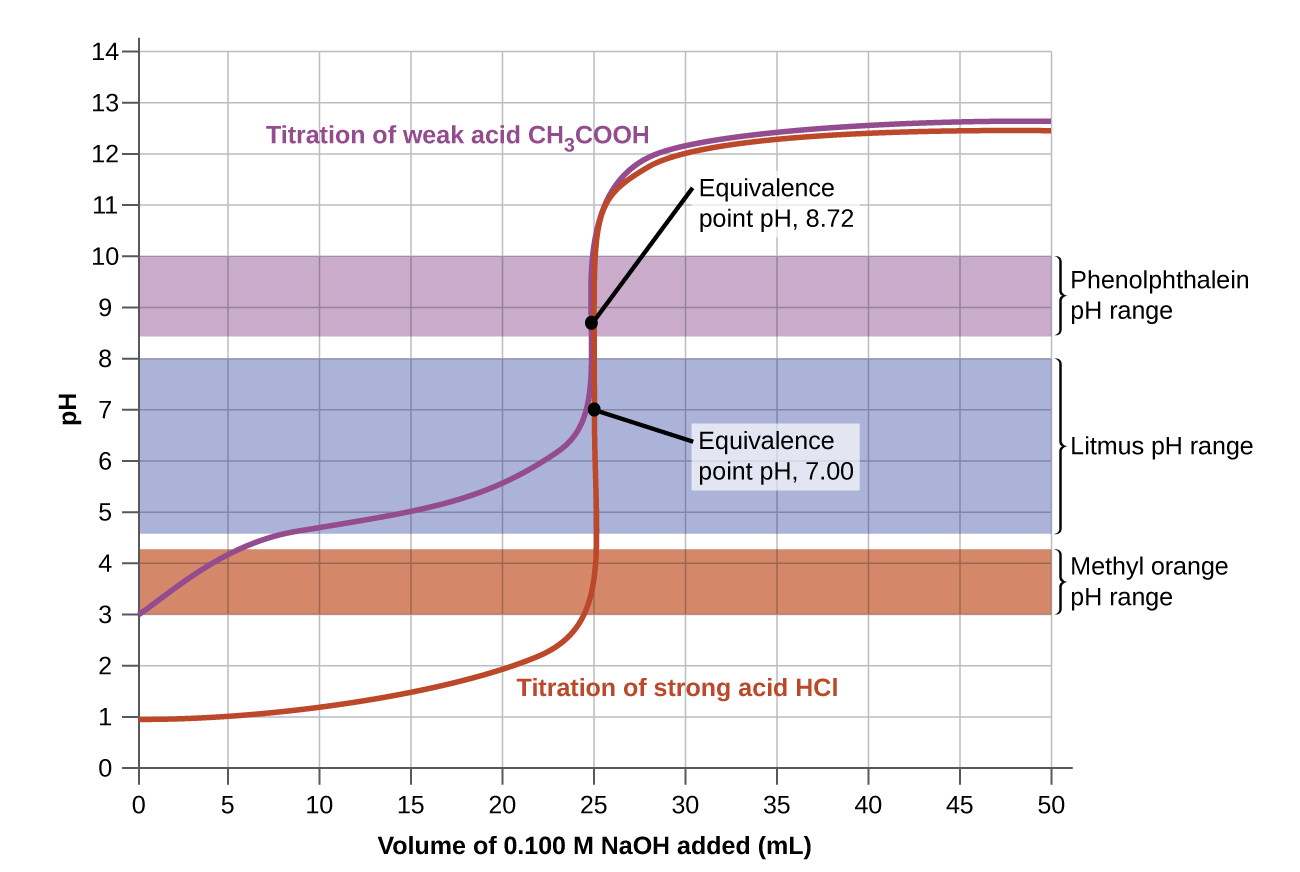
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax
How To Calculate Concentration From A Titration Curve A titration is a volumetric technique in which a solution of one. They can also be used to calculate the ph after a given point during a titration. Titration calculations are used to find the concentration of unknown solutions. By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. Example \ (\pageindex {3}\) : A titration is a volumetric technique in which a solution of one. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). The volume of the titrant added.
From gioetugkl.blob.core.windows.net
Titration Ncert at Gerald Young blog How To Calculate Concentration From A Titration Curve Example \ (\pageindex {3}\) : Suppose that a titration is performed and \(20.70 \: The volume of the titrant added. \ce{naoh}\) is required to reach the end point when titrated. By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. Titration calculations are. How To Calculate Concentration From A Titration Curve.
From www.chegg.com
Solved Use the following titration curve for the first four How To Calculate Concentration From A Titration Curve A titration is a volumetric technique in which a solution of one. By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. \ce{naoh}\) is required to reach the end point when titrated. Titration calculations are used to find the concentration of unknown solutions.. How To Calculate Concentration From A Titration Curve.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass How To Calculate Concentration From A Titration Curve By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. Suppose that a titration is performed and \(20.70 \: Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). \ce{naoh}\) is required to. How To Calculate Concentration From A Titration Curve.
From www.vrogue.co
7 Inspiration Titration Calculations Worksheet Gcse M vrogue.co How To Calculate Concentration From A Titration Curve The volume of the titrant added. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. Suppose that a titration is performed and. How To Calculate Concentration From A Titration Curve.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube How To Calculate Concentration From A Titration Curve Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). \ce{naoh}\) is required to reach the end point when titrated. Example \ (\pageindex {3}\) : They can also be used to calculate the ph after a given point during a titration. A titration is a volumetric technique in which a. How To Calculate Concentration From A Titration Curve.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp How To Calculate Concentration From A Titration Curve The volume of the titrant added. Suppose that a titration is performed and \(20.70 \: By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. They can also be used to calculate the ph after a given point during a titration. Let's assume. How To Calculate Concentration From A Titration Curve.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps How To Calculate Concentration From A Titration Curve They can also be used to calculate the ph after a given point during a titration. By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base. How To Calculate Concentration From A Titration Curve.
From medicine.ecu.edu
Titration of Antibody Protocol Flow Cytometry Core ECU How To Calculate Concentration From A Titration Curve Titration calculations are used to find the concentration of unknown solutions. A titration is a volumetric technique in which a solution of one. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0. How To Calculate Concentration From A Titration Curve.
From ar.inspiredpencil.com
Titration Curve Labeled How To Calculate Concentration From A Titration Curve Example \ (\pageindex {3}\) : They can also be used to calculate the ph after a given point during a titration. \ce{naoh}\) is required to reach the end point when titrated. Titration calculations are used to find the concentration of unknown solutions. Suppose that a titration is performed and \(20.70 \: By carefully measuring and recording the volume of titrant. How To Calculate Concentration From A Titration Curve.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog How To Calculate Concentration From A Titration Curve They can also be used to calculate the ph after a given point during a titration. Example \ (\pageindex {3}\) : Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \:. How To Calculate Concentration From A Titration Curve.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations How To Calculate Concentration From A Titration Curve By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. Suppose that a titration is performed and \(20.70 \: A titration is a volumetric technique in which a solution of one. Example \ (\pageindex {3}\) : The volume of the titrant added. They. How To Calculate Concentration From A Titration Curve.
From www.numerade.com
SOLVEDThe graph shows the titration curves for two How To Calculate Concentration From A Titration Curve Suppose that a titration is performed and \(20.70 \: Titration calculations are used to find the concentration of unknown solutions. The volume of the titrant added. Example \ (\pageindex {3}\) : They can also be used to calculate the ph after a given point during a titration. By carefully measuring and recording the volume of titrant required to reach this. How To Calculate Concentration From A Titration Curve.
From gioovibcc.blob.core.windows.net
Titration Gas Definition at Michael Hopkins blog How To Calculate Concentration From A Titration Curve Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. The volume of the titrant added. Suppose that a titration is performed and. How To Calculate Concentration From A Titration Curve.
From socratic.org
How to calculate the concentration of the acid solutions? Socratic How To Calculate Concentration From A Titration Curve By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. \ce{naoh}\) is required to reach the end point when titrated. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). They can also. How To Calculate Concentration From A Titration Curve.
From mmerevise.co.uk
pH Curves Questions and Revision MME How To Calculate Concentration From A Titration Curve The volume of the titrant added. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). They can also be used to calculate the ph after a given point during a titration. Example \ (\pageindex {3}\) : A titration is a volumetric technique in which a solution of one. Titration. How To Calculate Concentration From A Titration Curve.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration How To Calculate Concentration From A Titration Curve Titration calculations are used to find the concentration of unknown solutions. They can also be used to calculate the ph after a given point during a titration. Suppose that a titration is performed and \(20.70 \: Example \ (\pageindex {3}\) : A titration is a volumetric technique in which a solution of one. The volume of the titrant added. \ce{naoh}\). How To Calculate Concentration From A Titration Curve.
From education2research.com
Cracking the Code Unveiling the Titration Curves Worksheet Answers How To Calculate Concentration From A Titration Curve Example \ (\pageindex {3}\) : They can also be used to calculate the ph after a given point during a titration. A titration is a volumetric technique in which a solution of one. \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \: Titration calculations are used to find the concentration. How To Calculate Concentration From A Titration Curve.
From mungfali.com
Titration Curve Labeled How To Calculate Concentration From A Titration Curve By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. Suppose that a titration is performed and \(20.70 \: They can also be used to calculate the ph after a given point during a titration. \ce{naoh}\) is required to reach the end point. How To Calculate Concentration From A Titration Curve.
From courses.lumenlearning.com
AcidBase Titrations Chemistry How To Calculate Concentration From A Titration Curve A titration is a volumetric technique in which a solution of one. By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. They can also be used to calculate the ph after a given point during a titration. Example \ (\pageindex {3}\) :. How To Calculate Concentration From A Titration Curve.
From saylordotorg.github.io
AcidBase Titrations How To Calculate Concentration From A Titration Curve Suppose that a titration is performed and \(20.70 \: A titration is a volumetric technique in which a solution of one. Example \ (\pageindex {3}\) : \ce{naoh}\) is required to reach the end point when titrated. Titration calculations are used to find the concentration of unknown solutions. The volume of the titrant added. They can also be used to calculate. How To Calculate Concentration From A Titration Curve.
From mungfali.com
Endpoint Titration Curve How To Calculate Concentration From A Titration Curve Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to. How To Calculate Concentration From A Titration Curve.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax How To Calculate Concentration From A Titration Curve The volume of the titrant added. A titration is a volumetric technique in which a solution of one. They can also be used to calculate the ph after a given point during a titration. \ce{naoh}\) is required to reach the end point when titrated. Titration calculations are used to find the concentration of unknown solutions. Example \ (\pageindex {3}\) :. How To Calculate Concentration From A Titration Curve.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry How To Calculate Concentration From A Titration Curve A titration is a volumetric technique in which a solution of one. \ce{naoh}\) is required to reach the end point when titrated. Example \ (\pageindex {3}\) : Titration calculations are used to find the concentration of unknown solutions. They can also be used to calculate the ph after a given point during a titration. By carefully measuring and recording the. How To Calculate Concentration From A Titration Curve.
From hxettlyni.blob.core.windows.net
Titration Curve Line Of Best Fit at Rita Pinkerton blog How To Calculate Concentration From A Titration Curve Suppose that a titration is performed and \(20.70 \: By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. A titration is a volumetric technique in which a solution of one. Titration calculations are used to find the concentration of unknown solutions. Example. How To Calculate Concentration From A Titration Curve.
From giojtkyjh.blob.core.windows.net
Titration Calculation Steps at Ernest Blanton blog How To Calculate Concentration From A Titration Curve \ce{naoh}\) is required to reach the end point when titrated. The volume of the titrant added. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Suppose that a titration is performed and \(20.70 \: A titration is a volumetric technique in which a solution of one. Titration calculations are. How To Calculate Concentration From A Titration Curve.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts How To Calculate Concentration From A Titration Curve \ce{naoh}\) is required to reach the end point when titrated. Example \ (\pageindex {3}\) : Titration calculations are used to find the concentration of unknown solutions. By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. Suppose that a titration is performed and. How To Calculate Concentration From A Titration Curve.
From webmis.highland.cc.il.us
AcidBase Titrations How To Calculate Concentration From A Titration Curve Titration calculations are used to find the concentration of unknown solutions. A titration is a volumetric technique in which a solution of one. Suppose that a titration is performed and \(20.70 \: Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). The volume of the titrant added. \ce{naoh}\) is. How To Calculate Concentration From A Titration Curve.
From gioknvtfx.blob.core.windows.net
Lab Report On Acid Base Titration at Linda Crocker blog How To Calculate Concentration From A Titration Curve Example \ (\pageindex {3}\) : \ce{naoh}\) is required to reach the end point when titrated. The volume of the titrant added. A titration is a volumetric technique in which a solution of one. By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or.. How To Calculate Concentration From A Titration Curve.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation How To Calculate Concentration From A Titration Curve \ce{naoh}\) is required to reach the end point when titrated. Titration calculations are used to find the concentration of unknown solutions. They can also be used to calculate the ph after a given point during a titration. Suppose that a titration is performed and \(20.70 \: Example \ (\pageindex {3}\) : By carefully measuring and recording the volume of titrant. How To Calculate Concentration From A Titration Curve.
From giojtkyjh.blob.core.windows.net
Titration Calculation Steps at Ernest Blanton blog How To Calculate Concentration From A Titration Curve \ce{naoh}\) is required to reach the end point when titrated. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). A titration is a volumetric technique in which a solution of one. Titration calculations are used to find the concentration of unknown solutions. By carefully measuring and recording the volume. How To Calculate Concentration From A Titration Curve.
From www.vrogue.co
What Is The Chemical Equation For Titration Of Hcl Nh vrogue.co How To Calculate Concentration From A Titration Curve They can also be used to calculate the ph after a given point during a titration. Titration calculations are used to find the concentration of unknown solutions. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). A titration is a volumetric technique in which a solution of one. \ce{naoh}\). How To Calculate Concentration From A Titration Curve.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki How To Calculate Concentration From A Titration Curve Titration calculations are used to find the concentration of unknown solutions. The volume of the titrant added. By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. Example \ (\pageindex {3}\) : They can also be used to calculate the ph after a. How To Calculate Concentration From A Titration Curve.
From www.chegg.com
Solved Titration Curve for Titrating 0.300moULHC2H3O2(cog How To Calculate Concentration From A Titration Curve \ce{naoh}\) is required to reach the end point when titrated. Titration calculations are used to find the concentration of unknown solutions. A titration is a volumetric technique in which a solution of one. Suppose that a titration is performed and \(20.70 \: Example \ (\pageindex {3}\) : By carefully measuring and recording the volume of titrant required to reach this. How To Calculate Concentration From A Titration Curve.
From gionfqchy.blob.core.windows.net
How To Calculate Concentration Of Acetic Acid From Titration at Nancy How To Calculate Concentration From A Titration Curve Example \ (\pageindex {3}\) : A titration is a volumetric technique in which a solution of one. \ce{naoh}\) is required to reach the end point when titrated. By carefully measuring and recording the volume of titrant required to reach this endpoint, along with its known concentration, it is possible to calculate the concentration or. Titration calculations are used to find. How To Calculate Concentration From A Titration Curve.
From www.numerade.com
SOLVED Graph A was generated by titrating 25.00 mL of an unknown How To Calculate Concentration From A Titration Curve They can also be used to calculate the ph after a given point during a titration. A titration is a volumetric technique in which a solution of one. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required. How To Calculate Concentration From A Titration Curve.