Allyl Bromide Bromine Reaction . In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon. In allylic bromination, the br atom appears on the carbon next to the double bond: This reaction goes through a radical mechanism and it is. If plenty of hbr is present, then the radical. The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product and regenerate the bromine radical, which continues the radical chain. The reaction will occur on an allylic carbon (a carbon next to the alkene). Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products.
from www.numerade.com
If plenty of hbr is present, then the radical. This reaction goes through a radical mechanism and it is. The reaction will occur on an allylic carbon (a carbon next to the alkene). The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product and regenerate the bromine radical, which continues the radical chain. Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon. In allylic bromination, the br atom appears on the carbon next to the double bond:
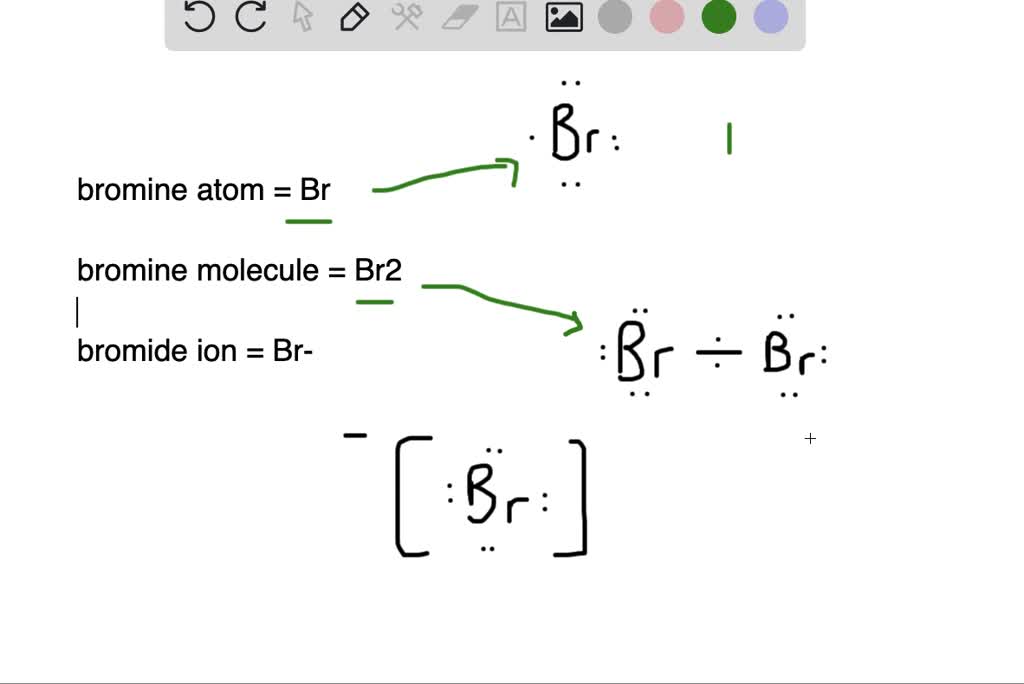
What is the difference between (a) a bromine atom, (b) a bromine
Allyl Bromide Bromine Reaction In allylic bromination, the br atom appears on the carbon next to the double bond: Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product and regenerate the bromine radical, which continues the radical chain. If plenty of hbr is present, then the radical. In allylic bromination, the br atom appears on the carbon next to the double bond: This reaction goes through a radical mechanism and it is. The reaction will occur on an allylic carbon (a carbon next to the alkene). In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Allyl Bromide Bromine Reaction This reaction goes through a radical mechanism and it is. The reaction will occur on an allylic carbon (a carbon next to the alkene). In allylic bromination, the br atom appears on the carbon next to the double bond: Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. The. Allyl Bromide Bromine Reaction.
From www.openwetware.org
ToddChem3x11 ToddL6 OpenWetWare Allyl Bromide Bromine Reaction In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon. If plenty of hbr is present, then the radical. Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. This reaction goes through a radical mechanism and it is. In allylic bromination, the. Allyl Bromide Bromine Reaction.
From slideplayer.com
Based on McMurry’s Organic Chemistry, 7th edition ppt download Allyl Bromide Bromine Reaction This reaction goes through a radical mechanism and it is. Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. In allylic bromination, the br atom appears on the carbon next to the double bond: If plenty of hbr is present, then the radical. The intermediate radical then reacts with. Allyl Bromide Bromine Reaction.
From www.researchgate.net
(a) Simplified scheme showing the formation of reactive bromine species Allyl Bromide Bromine Reaction In allylic bromination, the br atom appears on the carbon next to the double bond: In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon. If plenty of hbr is present, then the radical. The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product and regenerate the. Allyl Bromide Bromine Reaction.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Allyl Bromide Bromine Reaction Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. In allylic bromination, the br atom appears on the carbon next to the double bond: This reaction goes through a radical mechanism and it is. In that reaction, once br• adds to the pi bond, you obtain a radical on. Allyl Bromide Bromine Reaction.
From pubs.acs.org
CrossCoupling of Aromatic Bromides with Allylic Silanolate Salts Allyl Bromide Bromine Reaction The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product and regenerate the bromine radical, which continues the radical chain. In allylic bromination, the br atom appears on the carbon next to the double bond: Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as. Allyl Bromide Bromine Reaction.
From www.numerade.com
SOLVEDReaction of an allylic bromide with base produces conjugated Allyl Bromide Bromine Reaction In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon. In allylic bromination, the br atom appears on the carbon next to the double bond: The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product and regenerate the bromine radical, which continues the radical chain. Hydrogen halides. Allyl Bromide Bromine Reaction.
From www.chemtube3d.com
BaseCatalysed Bromination of Ketones Summary Allyl Bromide Bromine Reaction If plenty of hbr is present, then the radical. The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product and regenerate the bromine radical, which continues the radical chain. In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon. Hydrogen halides (hcl, hbr, and hi) react with. Allyl Bromide Bromine Reaction.
From www.masterorganicchemistry.com
What is Allylic Bromination? Master Organic Chemistry Allyl Bromide Bromine Reaction The reaction will occur on an allylic carbon (a carbon next to the alkene). This reaction goes through a radical mechanism and it is. Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product. Allyl Bromide Bromine Reaction.
From www.chemistrysteps.com
Allylic Bromination by NBS with Practice Problems Chemistry Steps Allyl Bromide Bromine Reaction Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. In allylic bromination, the br atom appears on the carbon next to the double bond: The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product and regenerate the bromine radical, which continues the radical. Allyl Bromide Bromine Reaction.
From app.jove.com
Radical Substitution Allylic Bromination Concept Organic Chemistry Allyl Bromide Bromine Reaction The reaction will occur on an allylic carbon (a carbon next to the alkene). In allylic bromination, the br atom appears on the carbon next to the double bond: Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. If plenty of hbr is present, then the radical. The intermediate. Allyl Bromide Bromine Reaction.
From pubs.rsc.org
DMSOallyl bromide a mild and efficient reagent for atom economic one Allyl Bromide Bromine Reaction If plenty of hbr is present, then the radical. The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product and regenerate the bromine radical, which continues the radical chain. The reaction will occur on an allylic carbon (a carbon next to the alkene). In that reaction, once br• adds to the pi bond, you. Allyl Bromide Bromine Reaction.
From slideplayer.com
Alkyl Halides 23 May ppt download Allyl Bromide Bromine Reaction The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product and regenerate the bromine radical, which continues the radical chain. This reaction goes through a radical mechanism and it is. In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon. Hydrogen halides (hcl, hbr, and hi) react. Allyl Bromide Bromine Reaction.
From www.masterorganicchemistry.com
Bromination of alkenes with Br2 to give dibromides Master Organic Allyl Bromide Bromine Reaction The reaction will occur on an allylic carbon (a carbon next to the alkene). Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. This reaction goes through a radical mechanism and it is. In allylic bromination, the br atom appears on the carbon next to the double bond: The. Allyl Bromide Bromine Reaction.
From www.chemistrysteps.com
Allylic Bromination by NBS with Practice Problems Chemistry Steps Allyl Bromide Bromine Reaction The reaction will occur on an allylic carbon (a carbon next to the alkene). In allylic bromination, the br atom appears on the carbon next to the double bond: This reaction goes through a radical mechanism and it is. If plenty of hbr is present, then the radical. In that reaction, once br• adds to the pi bond, you obtain. Allyl Bromide Bromine Reaction.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Allyl Bromide Bromine Reaction This reaction goes through a radical mechanism and it is. In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon. Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. The reaction will occur on an allylic carbon (a carbon next to the. Allyl Bromide Bromine Reaction.
From www.chemistrysteps.com
Allylic Bromination by NBS with Practice Problems Chemistry Steps Allyl Bromide Bromine Reaction This reaction goes through a radical mechanism and it is. The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product and regenerate the bromine radical, which continues the radical chain. If plenty of hbr is present, then the radical. The reaction will occur on an allylic carbon (a carbon next to the alkene). In. Allyl Bromide Bromine Reaction.
From www.researchgate.net
Scheme 3 Synthesis of allylic bromide 6. Download Scientific Diagram Allyl Bromide Bromine Reaction Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. This reaction goes through a radical mechanism and it is. The reaction will occur on an allylic carbon (a carbon next to the alkene). In that reaction, once br• adds to the pi bond, you obtain a radical on a. Allyl Bromide Bromine Reaction.
From www.numerade.com
SOLVED Allylic Bromination review) Mechanistic Example Br Allyl Bromide Bromine Reaction Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. If plenty of hbr is present, then the radical. In allylic bromination, the br atom appears on the carbon next to the double bond: The reaction will occur on an allylic carbon (a carbon next to the alkene). The intermediate. Allyl Bromide Bromine Reaction.
From chemizi.blogspot.com
Which one is more reactive between vinyl bromide and allyl bromide in Allyl Bromide Bromine Reaction In allylic bromination, the br atom appears on the carbon next to the double bond: Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. If plenty of hbr is present, then the radical. The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product. Allyl Bromide Bromine Reaction.
From www.masterorganicchemistry.com
NBromoSuccinimide (NBS) As A Reagent In Organic Chemistry Allyl Bromide Bromine Reaction Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. If plenty of hbr is present, then the radical. In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon. The reaction will occur on an allylic carbon (a carbon next to the alkene).. Allyl Bromide Bromine Reaction.
From www.youtube.com
(A) In allylic substitution propene gives allyl bromide. (R) NBS is a Allyl Bromide Bromine Reaction The reaction will occur on an allylic carbon (a carbon next to the alkene). In allylic bromination, the br atom appears on the carbon next to the double bond: This reaction goes through a radical mechanism and it is. In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon. The intermediate radical then. Allyl Bromide Bromine Reaction.
From www.chegg.com
Solved Allylic bromide 2 was recently used as a key fragment Allyl Bromide Bromine Reaction This reaction goes through a radical mechanism and it is. In allylic bromination, the br atom appears on the carbon next to the double bond: The reaction will occur on an allylic carbon (a carbon next to the alkene). The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product and regenerate the bromine radical,. Allyl Bromide Bromine Reaction.
From www.chegg.com
Solved H H Br H CIPd allyl bromide ClPd + In H + I ??? CI Allyl Bromide Bromine Reaction In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon. If plenty of hbr is present, then the radical. The reaction will occur on an allylic carbon (a carbon next to the alkene). The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product and regenerate the bromine. Allyl Bromide Bromine Reaction.
From www.chegg.com
Solved Reaction of an allylic bromide with base produces a Allyl Bromide Bromine Reaction Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. In allylic bromination, the br atom appears on the carbon next to the double bond: In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon. The intermediate radical then reacts with a br. Allyl Bromide Bromine Reaction.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Allyl Bromide Bromine Reaction This reaction goes through a radical mechanism and it is. If plenty of hbr is present, then the radical. In allylic bromination, the br atom appears on the carbon next to the double bond: The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product and regenerate the bromine radical, which continues the radical chain.. Allyl Bromide Bromine Reaction.
From www.slideserve.com
PPT 10. Organohalides PowerPoint Presentation, free download ID149791 Allyl Bromide Bromine Reaction If plenty of hbr is present, then the radical. In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon. This reaction goes through a radical mechanism and it is. The reaction will occur on an allylic carbon (a carbon next to the alkene). Hydrogen halides (hcl, hbr, and hi) react with alkenes in. Allyl Bromide Bromine Reaction.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Allyl Bromide Bromine Reaction In allylic bromination, the br atom appears on the carbon next to the double bond: In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon. Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. The intermediate radical then reacts with a br. Allyl Bromide Bromine Reaction.
From slideplayer.com
Chapter 10 Organohalides ppt download Allyl Bromide Bromine Reaction This reaction goes through a radical mechanism and it is. In allylic bromination, the br atom appears on the carbon next to the double bond: Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide. Allyl Bromide Bromine Reaction.
From www.chegg.com
Chemistry Archive June 20, 2017 Allyl Bromide Bromine Reaction The reaction will occur on an allylic carbon (a carbon next to the alkene). This reaction goes through a radical mechanism and it is. In allylic bromination, the br atom appears on the carbon next to the double bond: If plenty of hbr is present, then the radical. In that reaction, once br• adds to the pi bond, you obtain. Allyl Bromide Bromine Reaction.
From www.sielc.com
Allyl bromide SIELC Allyl Bromide Bromine Reaction The reaction will occur on an allylic carbon (a carbon next to the alkene). In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon. Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. If plenty of hbr is present, then the radical.. Allyl Bromide Bromine Reaction.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Allyl Bromide Bromine Reaction If plenty of hbr is present, then the radical. The reaction will occur on an allylic carbon (a carbon next to the alkene). In that reaction, once br• adds to the pi bond, you obtain a radical on a carbon. This reaction goes through a radical mechanism and it is. In allylic bromination, the br atom appears on the carbon. Allyl Bromide Bromine Reaction.
From www.youtube.com
Allylic Bromination of Alkenes YouTube Allyl Bromide Bromine Reaction Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. If plenty of hbr is present, then the radical. The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product and regenerate the bromine radical, which continues the radical chain. The reaction will occur on. Allyl Bromide Bromine Reaction.
From www.chemistrysteps.com
Allylic Bromination by NBS with Practice Problems Chemistry Steps Allyl Bromide Bromine Reaction This reaction goes through a radical mechanism and it is. Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. The intermediate radical then reacts with a br 2 molecule to generate the allylic bromide product and regenerate the bromine radical, which continues the radical chain. In allylic bromination, the. Allyl Bromide Bromine Reaction.
From www.slideserve.com
PPT 10. Organohalides PowerPoint Presentation, free download ID3365963 Allyl Bromide Bromine Reaction In allylic bromination, the br atom appears on the carbon next to the double bond: This reaction goes through a radical mechanism and it is. The reaction will occur on an allylic carbon (a carbon next to the alkene). Hydrogen halides (hcl, hbr, and hi) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. If. Allyl Bromide Bromine Reaction.