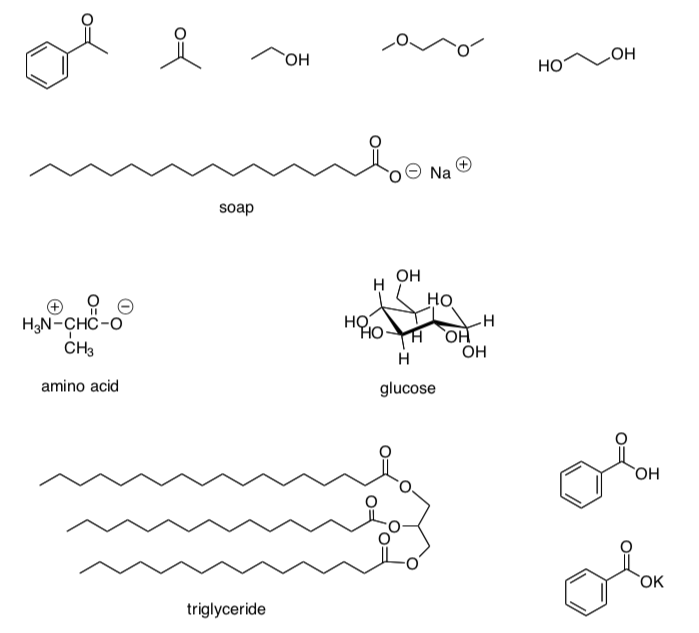
Oil Imf Chemistry . Intermolecular forces are attractive forces between separate molecules. But when you pour syrup on pancakes or add oil to a car engine, you note that syrup. Cooking oil and water are used to illustrate that polar. Identify the cohesive forces in the motor oil. 11.1 oil, water, and dish soap (intermolecular forces i) subjects: Properties of liquids, intermolecular forces. All of the attractive forces between. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. When you pour a glass of water, or fill a car with gasoline, you observe that water and gasoline flow freely. Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. Determine whether the forces interact with the surface of glass.
from chem.libretexts.org
When you pour a glass of water, or fill a car with gasoline, you observe that water and gasoline flow freely. Cooking oil and water are used to illustrate that polar. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). Properties of liquids, intermolecular forces. Identify the cohesive forces in the motor oil. All of the attractive forces between. 11.1 oil, water, and dish soap (intermolecular forces i) subjects: Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. Determine whether the forces interact with the surface of glass.
14 IMF Chemistry LibreTexts
Oil Imf Chemistry The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. When you pour a glass of water, or fill a car with gasoline, you observe that water and gasoline flow freely. But when you pour syrup on pancakes or add oil to a car engine, you note that syrup. Cooking oil and water are used to illustrate that polar. 11.1 oil, water, and dish soap (intermolecular forces i) subjects: Determine whether the forces interact with the surface of glass. Intermolecular forces are attractive forces between separate molecules. Identify the cohesive forces in the motor oil. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. All of the attractive forces between. Properties of liquids, intermolecular forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Oil Imf Chemistry Intermolecular forces are attractive forces between separate molecules. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. Identify the cohesive forces in the motor oil. Cooking oil and water are. Oil Imf Chemistry.
From chem.libretexts.org
14 IMF Chemistry LibreTexts Oil Imf Chemistry Cooking oil and water are used to illustrate that polar. When you pour a glass of water, or fill a car with gasoline, you observe that water and gasoline flow freely. Identify the cohesive forces in the motor oil. Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. 11.1 oil, water, and dish soap (intermolecular forces i) subjects: All. Oil Imf Chemistry.
From igcsetuition.blogspot.com
O Level Chemistry Oil Refining Crude Oil Oil Imf Chemistry Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). When you pour a glass of water, or fill a car with gasoline, you observe that water and. Oil Imf Chemistry.
From www.edibleoilrefinerymachine.com
Edible Oil Physical Refining vs Edible Oil Chemical Refining_Tech Oil Imf Chemistry But when you pour syrup on pancakes or add oil to a car engine, you note that syrup. Identify the cohesive forces in the motor oil. Determine whether the forces interact with the surface of glass. Cooking oil and water are used to illustrate that polar. All of the attractive forces between. Intermolecular forces are attractive forces between separate molecules.. Oil Imf Chemistry.
From spmchemistry.blog.onlinetuition.com.my
Fats and Oils SPM Chemistry Oil Imf Chemistry All of the attractive forces between. But when you pour syrup on pancakes or add oil to a car engine, you note that syrup. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). Determine whether the forces interact with the. Oil Imf Chemistry.
From www.youtube.com
How to Determine the Strength of Intermolecular Forces (IMFs) and Rank Oil Imf Chemistry Properties of liquids, intermolecular forces. 11.1 oil, water, and dish soap (intermolecular forces i) subjects: Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. Cooking oil and water are used to illustrate that polar. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous. Oil Imf Chemistry.
From openpress.usask.ca
2.4. Effects of Intermolecular Forces Introduction to Organic Chemistry Oil Imf Chemistry Determine whether the forces interact with the surface of glass. Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. All of the attractive forces between. Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. Properties of liquids, intermolecular forces. Intermolecular forces are attractive forces between separate molecules. The type of intermolecular forces (imfs) exhibited by. Oil Imf Chemistry.
From chem.libretexts.org
14 IMF Chemistry LibreTexts Oil Imf Chemistry But when you pour syrup on pancakes or add oil to a car engine, you note that syrup. Intermolecular forces are attractive forces between separate molecules. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). Determine whether the forces interact. Oil Imf Chemistry.
From chem.libretexts.org
2.11 Intermolecular Forces and Relative Boiling Points (bp Oil Imf Chemistry All of the attractive forces between. 11.1 oil, water, and dish soap (intermolecular forces i) subjects: Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. Cooking oil and water are used to illustrate that polar. Identify the cohesive forces in the motor oil. Determine whether the forces interact with the surface of glass. Intermolecular forces hold multiple molecules together. Oil Imf Chemistry.
From www.thecosmeticchemist.com
The Cosmetic Chemist Oil Imf Chemistry Identify the cohesive forces in the motor oil. All of the attractive forces between. Intermolecular forces are attractive forces between separate molecules. Properties of liquids, intermolecular forces. 11.1 oil, water, and dish soap (intermolecular forces i) subjects: When you pour a glass of water, or fill a car with gasoline, you observe that water and gasoline flow freely. But when. Oil Imf Chemistry.
From www.intechopen.com
Chemical Structure, Quality Indices and Bioactivity of Essential Oil Oil Imf Chemistry Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. Intermolecular forces are attractive forces between separate molecules. Cooking oil and water are used to illustrate that polar. All of the attractive forces between. Identify the cohesive forces in the motor oil. 11.1 oil, water, and dish soap (intermolecular forces i) subjects: Determine whether the forces interact with the surface. Oil Imf Chemistry.
From wagine.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Oil Imf Chemistry Cooking oil and water are used to illustrate that polar. Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. All of the attractive forces between. When you pour a glass of water, or fill a car with gasoline, you observe that water and gasoline flow freely.. Oil Imf Chemistry.
From www.youtube.com
AP Chemistry REview of IMF Form YouTube Oil Imf Chemistry Cooking oil and water are used to illustrate that polar. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). When you pour a glass of water, or fill a car with gasoline, you observe that water and gasoline flow freely.. Oil Imf Chemistry.
From sherpa-online.com
GCSE Chemistry Crude Oil and Hydrocarbons, what is Crude Oil and how Oil Imf Chemistry Intermolecular forces are attractive forces between separate molecules. Identify the cohesive forces in the motor oil. But when you pour syrup on pancakes or add oil to a car engine, you note that syrup. All of the attractive forces between. When you pour a glass of water, or fill a car with gasoline, you observe that water and gasoline flow. Oil Imf Chemistry.
From www.dreamstime.com
Oil composition stock vector. Illustration of ring, atoms 64171764 Oil Imf Chemistry Properties of liquids, intermolecular forces. 11.1 oil, water, and dish soap (intermolecular forces i) subjects: When you pour a glass of water, or fill a car with gasoline, you observe that water and gasoline flow freely. Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. Cooking oil and water are used to illustrate that polar. But. Oil Imf Chemistry.
From www.studocu.com
Bonding and IMF Test Review Chemistry Bonding and IMF Test Review A Oil Imf Chemistry Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. Properties of liquids, intermolecular forces. 11.1 oil, water, and dish soap (intermolecular forces i) subjects: Identify the cohesive forces in the motor oil. Determine whether the forces interact with the surface of glass. The type of intermolecular. Oil Imf Chemistry.
From www.ic2r.com
Oil Chemistry IC2R Ltd Oil Imf Chemistry Determine whether the forces interact with the surface of glass. Intermolecular forces are attractive forces between separate molecules. All of the attractive forces between. 11.1 oil, water, and dish soap (intermolecular forces i) subjects: The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution. Oil Imf Chemistry.
From www.pinterest.com
GCSE Bitesize Fractional distillation Fractional distillation, Gcse Oil Imf Chemistry But when you pour syrup on pancakes or add oil to a car engine, you note that syrup. Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). 11.1 oil, water,. Oil Imf Chemistry.
From www.organicchemistrytutor.com
Intermolecular Forces in Organic Chemistry — Organic Chemistry Tutor Oil Imf Chemistry Cooking oil and water are used to illustrate that polar. Determine whether the forces interact with the surface of glass. But when you pour syrup on pancakes or add oil to a car engine, you note that syrup. Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. Intermolecular forces hold multiple molecules together and determine many of a substance’s. Oil Imf Chemistry.
From crudeoilpeak.info
IMF warns of oil scarcity and a 60 oil price increase within a year Oil Imf Chemistry 11.1 oil, water, and dish soap (intermolecular forces i) subjects: Properties of liquids, intermolecular forces. Intermolecular forces are attractive forces between separate molecules. Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a. Oil Imf Chemistry.
From crudeoilpeak.info
IMF warns of oil scarcity and a 60 oil price increase within a year Oil Imf Chemistry Cooking oil and water are used to illustrate that polar. 11.1 oil, water, and dish soap (intermolecular forces i) subjects: When you pour a glass of water, or fill a car with gasoline, you observe that water and gasoline flow freely. But when you pour syrup on pancakes or add oil to a car engine, you note that syrup. Determine. Oil Imf Chemistry.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Oil Imf Chemistry Cooking oil and water are used to illustrate that polar. Identify the cohesive forces in the motor oil. Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. 11.1 oil, water, and dish soap (intermolecular forces i) subjects: But when you pour syrup on pancakes or add oil to a car engine, you note that syrup. The. Oil Imf Chemistry.
From www.researchgate.net
Chemical formulae of some essential oils' constituents. Download Oil Imf Chemistry All of the attractive forces between. Cooking oil and water are used to illustrate that polar. Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. 11.1 oil, water, and dish soap (intermolecular forces i) subjects: But when you pour syrup on pancakes or add oil to a car engine, you note that syrup. The type of. Oil Imf Chemistry.
From andi-healthy.blogspot.com
Vegetable Oil Molecular Structure Andi Healthy Oil Imf Chemistry The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). All of the attractive forces between. Identify the cohesive forces in the motor oil. Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. 11.1 oil, water, and dish soap (intermolecular. Oil Imf Chemistry.
From chem.libretexts.org
17.2 Fats and Oils Chemistry LibreTexts Oil Imf Chemistry Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. All of the attractive forces between. Cooking oil and water are used to illustrate that polar. Intermolecular forces are attractive forces between separate molecules. When you pour a glass of water, or fill a car with gasoline, you observe that water and gasoline flow freely. Determine whether. Oil Imf Chemistry.
From www.slideserve.com
PPT Covalent Compounds PowerPoint Presentation, free download ID Oil Imf Chemistry Identify the cohesive forces in the motor oil. Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. 11.1 oil, water, and dish soap (intermolecular forces i) subjects: Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. Cooking oil and water are used to illustrate that polar. Properties of liquids, intermolecular forces. Determine whether the forces. Oil Imf Chemistry.
From www.slideserve.com
PPT Unit 13 Intermolecular Forces/Solids, Liquids and Solutions Oil Imf Chemistry Properties of liquids, intermolecular forces. 11.1 oil, water, and dish soap (intermolecular forces i) subjects: Determine whether the forces interact with the surface of glass. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). All of the attractive forces between.. Oil Imf Chemistry.
From mavink.com
Chemical Structure Of Oil Oil Imf Chemistry But when you pour syrup on pancakes or add oil to a car engine, you note that syrup. Determine whether the forces interact with the surface of glass. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). All of the. Oil Imf Chemistry.
From chem.libretexts.org
17.2 Fats and Oils Chemistry LibreTexts Oil Imf Chemistry Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). Cooking oil and water are used to illustrate that polar. Identify the cohesive forces in the motor oil.. Oil Imf Chemistry.
From www.slideserve.com
PPT Molecular Shapes for Liquids and IMF Chem 2 PowerPoint Oil Imf Chemistry Identify the cohesive forces in the motor oil. Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. When you pour a glass of water, or fill a car with gasoline, you observe that water and gasoline flow freely. Cooking oil and water are used to illustrate. Oil Imf Chemistry.
From chem.libretexts.org
14 IMF Chemistry LibreTexts Oil Imf Chemistry Properties of liquids, intermolecular forces. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). But when you pour syrup on pancakes or add oil to a car engine, you note that syrup. Cooking oil and water are used to illustrate. Oil Imf Chemistry.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Oil Imf Chemistry Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. All of the attractive forces between. Identify the cohesive forces in the motor oil. Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form. Oil Imf Chemistry.
From accuraclabs.com
Oils & Lubricant Testing Physical and Chemical analysis of all kinds Oil Imf Chemistry The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). But when you pour syrup on pancakes or add oil to a car engine, you note that syrup. Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. All of the. Oil Imf Chemistry.
From www.youtube.com
How to Determine the Types of Intermolecular Forces (IMFs) QUICK Oil Imf Chemistry Properties of liquids, intermolecular forces. Intermolecular forces or imfs are attractive and repulsive electromagnetic forces. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution (soluble or miscible). Determine whether the forces interact with the surface of glass. Intermolecular forces are attractive forces between. Oil Imf Chemistry.
From za.pinterest.com
Teaching Chemistry, Chemistry Lessons, Science Chemistry, Ag Science Oil Imf Chemistry 11.1 oil, water, and dish soap (intermolecular forces i) subjects: Intermolecular forces are attractive forces between separate molecules. Cooking oil and water are used to illustrate that polar. All of the attractive forces between. The type of intermolecular forces (imfs) exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution. Oil Imf Chemistry.