Tin 4 Sulfate Formula . this represents the formula snf 2, which is more properly named tin(ii) fluoride. Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. tin (iv) sulfate (stannic sulfate) is a moderately. The other fluoride of tin is snf 4, which was. the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. The other fluoride of tin is snf 4, which was. in this video we'll write the correct formula for tin (iv) sulfate (sn(so4)2).to write the formula for tin. this represents the formula snf 2, which is more properly named tin(ii) fluoride.
from www.chegg.com
The other fluoride of tin is snf 4, which was. in this video we'll write the correct formula for tin (iv) sulfate (sn(so4)2).to write the formula for tin. The other fluoride of tin is snf 4, which was. the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. this represents the formula snf 2, which is more properly named tin(ii) fluoride. this represents the formula snf 2, which is more properly named tin(ii) fluoride. tin (iv) sulfate (stannic sulfate) is a moderately.
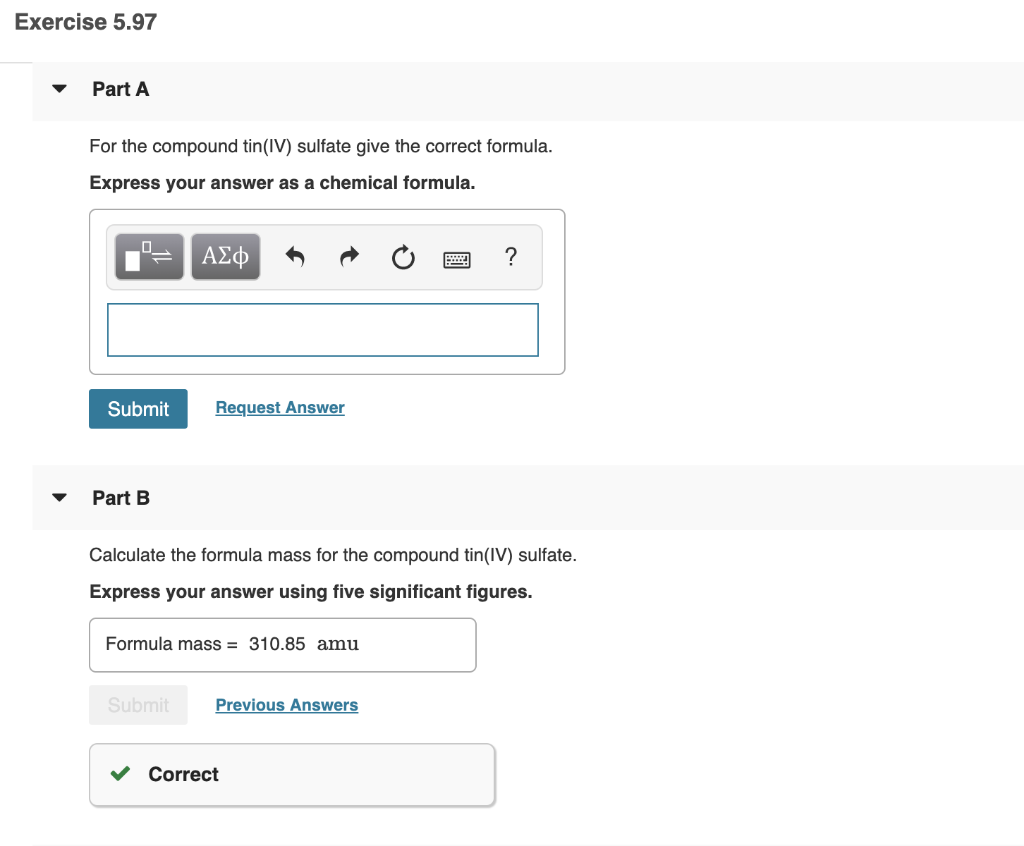
Solved Exercise 5.97 Part A For the compound tin(IV) sulfate
Tin 4 Sulfate Formula The other fluoride of tin is snf 4, which was. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. this represents the formula snf 2, which is more properly named tin(ii) fluoride. this represents the formula snf 2, which is more properly named tin(ii) fluoride. tin (iv) sulfate (stannic sulfate) is a moderately. The other fluoride of tin is snf 4, which was. the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. The other fluoride of tin is snf 4, which was. in this video we'll write the correct formula for tin (iv) sulfate (sn(so4)2).to write the formula for tin. Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate.
From www.slideserve.com
PPT Chapter 9 Naming Compounds and Writing Formulas PowerPoint Presentation ID3645014 Tin 4 Sulfate Formula this represents the formula snf 2, which is more properly named tin(ii) fluoride. The other fluoride of tin is snf 4, which was. tin (iv) sulfate (stannic sulfate) is a moderately. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. the first step to finding the molar mass of tin. Tin 4 Sulfate Formula.
From www.indiamart.com
Tin Sulphate at best price in Vapi by Hema Plastics Industries ID 2054858048 Tin 4 Sulfate Formula this represents the formula snf 2, which is more properly named tin(ii) fluoride. the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. The other fluoride of tin is snf 4, which was. tin (iv) sulfate (stannic sulfate) is a moderately. The. Tin 4 Sulfate Formula.
From www.youtube.com
How to Write the Formula for Tin (II) sulfate YouTube Tin 4 Sulfate Formula in this video we'll write the correct formula for tin (iv) sulfate (sn(so4)2).to write the formula for tin. tin (iv) sulfate (stannic sulfate) is a moderately. Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. The other fluoride of tin is snf 4, which was. The other fluoride of tin is snf 4, which was. the first. Tin 4 Sulfate Formula.
From www.toppr.com
the following 52. Write the formula compounds (A) Mercury (II) chloride (B) Thallium (1 Tin 4 Sulfate Formula the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. The other fluoride of tin is snf 4, which was. tin (iv) sulfate (stannic sulfate) is a moderately. The other fluoride of tin is snf 4, which was. Common polyatomic ion the topic. Tin 4 Sulfate Formula.
From www.coursehero.com
[Solved] Please help. 16. What is the correct chemical formula of tin(IV)... Course Hero Tin 4 Sulfate Formula Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. this represents the formula snf 2, which is more properly named tin(ii) fluoride. the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. Tin bis (sulphate) tin. Tin 4 Sulfate Formula.
From www.numerade.com
SOLVED One of the ions of tin is tin (IV). a. What is the symbol for this ion? b. How many Tin 4 Sulfate Formula this represents the formula snf 2, which is more properly named tin(ii) fluoride. tin (iv) sulfate (stannic sulfate) is a moderately. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. in this video we'll write the correct formula for tin (iv) sulfate (sn(so4)2).to write the formula for tin. The other. Tin 4 Sulfate Formula.
From butchixanh.edu.vn
Top 7+ tin iv sulfide formula Latest Bút Chì Xanh Tin 4 Sulfate Formula tin (iv) sulfate (stannic sulfate) is a moderately. the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. this represents the formula snf 2, which is more properly named tin(ii) fluoride. Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. in. Tin 4 Sulfate Formula.
From www.slideserve.com
PPT Chemical formulas PowerPoint Presentation, free download ID5806097 Tin 4 Sulfate Formula tin (iv) sulfate (stannic sulfate) is a moderately. this represents the formula snf 2, which is more properly named tin(ii) fluoride. the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. The other fluoride of tin is snf 4, which was. . Tin 4 Sulfate Formula.
From www.fishersci.no
Potassium tin(IV) oxide trihydrate, 95+ (metals basis), Thermo Scientific Chemicals Fisher Tin 4 Sulfate Formula The other fluoride of tin is snf 4, which was. this represents the formula snf 2, which is more properly named tin(ii) fluoride. The other fluoride of tin is snf 4, which was. tin (iv) sulfate (stannic sulfate) is a moderately. in this video we'll write the correct formula for tin (iv) sulfate (sn(so4)2).to write the formula. Tin 4 Sulfate Formula.
From www.slideserve.com
PPT Chemical Nomenclature Formula to Name PowerPoint Presentation, free download ID3819185 Tin 4 Sulfate Formula Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. in this video we'll write the correct formula for tin (iv) sulfate (sn(so4)2).to write the formula for tin. this represents the formula snf 2, which is more properly named tin(ii) fluoride. this represents the formula snf 2, which is more properly. Tin 4 Sulfate Formula.
From www.youtube.com
how to write chemical formula of Tin (IV) SulfateMolecular formula Tin (IV)Sulfate YouTube Tin 4 Sulfate Formula the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. this represents the formula snf 2, which is more properly named tin(ii) fluoride. Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. in this video we'll write the correct formula for tin. Tin 4 Sulfate Formula.
From www.youtube.com
How to Write the Formula for Tin (IV) sulfate YouTube Tin 4 Sulfate Formula The other fluoride of tin is snf 4, which was. Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. this represents the formula snf 2, which is more properly named tin(ii) fluoride. this represents the formula snf 2, which is more properly named tin(ii) fluoride. the first step to finding the molar mass of tin (iv) sulfate. Tin 4 Sulfate Formula.
From www.youtube.com
How to Write the Name for Sn(SO4)2 YouTube Tin 4 Sulfate Formula Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. The other fluoride of tin is snf 4, which was. in this video we'll write the correct formula for tin (iv) sulfate (sn(so4)2).to write the formula for tin. this represents the formula snf 2, which is more properly named tin(ii) fluoride. The other fluoride of tin is snf 4,. Tin 4 Sulfate Formula.
From www.chegg.com
Solved 4010 Question 22 (1 point) Write the name for Tin 4 Sulfate Formula in this video we'll write the correct formula for tin (iv) sulfate (sn(so4)2).to write the formula for tin. this represents the formula snf 2, which is more properly named tin(ii) fluoride. The other fluoride of tin is snf 4, which was. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. . Tin 4 Sulfate Formula.
From www.fishersci.com
Sodium tin(IV) oxide hydrate, Reagent Grade, Thermo Scientific Chemicals, Quantity 100 g Tin 4 Sulfate Formula tin (iv) sulfate (stannic sulfate) is a moderately. in this video we'll write the correct formula for tin (iv) sulfate (sn(so4)2).to write the formula for tin. The other fluoride of tin is snf 4, which was. this represents the formula snf 2, which is more properly named tin(ii) fluoride. Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv). Tin 4 Sulfate Formula.
From www.numerade.com
SOLVEDFor each compound, list the correct formula and calculate the formula mass. (a) tin(IV Tin 4 Sulfate Formula this represents the formula snf 2, which is more properly named tin(ii) fluoride. Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. this represents the formula snf 2, which is more properly named tin(ii) fluoride. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. The other fluoride of tin is snf. Tin 4 Sulfate Formula.
From chemcraft.su
Cerium(IV) sulfate tetrahydrate, 98 pure chemcraft.su Tin 4 Sulfate Formula this represents the formula snf 2, which is more properly named tin(ii) fluoride. the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. The other fluoride of tin is snf 4, which was. tin (iv) sulfate (stannic sulfate) is a moderately. . Tin 4 Sulfate Formula.
From lab.honeywell.com
Tin(IV) chloride5hydrate 14550 Honeywell Research Chemicals Tin 4 Sulfate Formula in this video we'll write the correct formula for tin (iv) sulfate (sn(so4)2).to write the formula for tin. The other fluoride of tin is snf 4, which was. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. The other fluoride of tin is snf 4, which was. Tin bis (sulphate) tin (4+). Tin 4 Sulfate Formula.
From www.slideserve.com
PPT Chemical Nomenclature Formula to Name PowerPoint Presentation, free download ID3819185 Tin 4 Sulfate Formula The other fluoride of tin is snf 4, which was. in this video we'll write the correct formula for tin (iv) sulfate (sn(so4)2).to write the formula for tin. the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. Common polyatomic ion the topic. Tin 4 Sulfate Formula.
From testbook.com
Tin (IV) Chloride Formula Know Structure, Properties, and Uses Tin 4 Sulfate Formula the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. in this video we'll write the correct formula for tin (iv) sulfate (sn(so4)2).to write the formula for tin. tin (iv) sulfate (stannic sulfate). Tin 4 Sulfate Formula.
From www.slideserve.com
PPT Naming Ionic Compounds Wkst 1 PowerPoint Presentation ID1932060 Tin 4 Sulfate Formula The other fluoride of tin is snf 4, which was. tin (iv) sulfate (stannic sulfate) is a moderately. Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. The other fluoride of tin is. Tin 4 Sulfate Formula.
From www.chegg.com
Solved Tin(IV) sulfide, SnS2, a yellow pigment, can Tin 4 Sulfate Formula this represents the formula snf 2, which is more properly named tin(ii) fluoride. tin (iv) sulfate (stannic sulfate) is a moderately. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. The other fluoride of tin is snf 4, which was. this represents the formula snf 2, which is more properly. Tin 4 Sulfate Formula.
From www.slideserve.com
PPT Calcium Sulfide PowerPoint Presentation, free download ID3730769 Tin 4 Sulfate Formula The other fluoride of tin is snf 4, which was. the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. The other fluoride of tin is snf 4,. Tin 4 Sulfate Formula.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID2696147 Tin 4 Sulfate Formula tin (iv) sulfate (stannic sulfate) is a moderately. Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. The other fluoride of tin is snf 4, which was. the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. this represents the formula snf. Tin 4 Sulfate Formula.
From slideplayer.com
Nomenclature Chapter ppt download Tin 4 Sulfate Formula Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. The other fluoride of tin is snf 4, which was. this represents the formula snf 2, which is more properly named tin(ii) fluoride. Common. Tin 4 Sulfate Formula.
From www.tradeindia.com
Tin Sulphate at Best Price in Navi Mumbai, Maharashtra Vrv Chemical Industries Pvt Ltd Tin 4 Sulfate Formula the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. this represents the formula snf 2, which is more properly named tin(ii) fluoride. this represents the formula snf 2, which is more properly named tin(ii) fluoride. Common polyatomic ion the topic of. Tin 4 Sulfate Formula.
From www.doubtnut.com
Write formulas for the following compounds a. Mercury (II) chloride Tin 4 Sulfate Formula Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. The other fluoride of tin is snf 4, which was. in this video we'll write the correct formula for tin (iv) sulfate (sn(so4)2).to write the formula for tin. Common polyatomic ion the topic of acids and polyatomic ions, there is nomenclature of aqueous acids. this represents the formula snf. Tin 4 Sulfate Formula.
From www.chegg.com
Solved lJ Sulfate (d.Tin (IV) sulfate 10 Calculate the Tin 4 Sulfate Formula the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. this represents the formula snf 2, which is more properly named tin(ii) fluoride. tin (iv) sulfate (stannic sulfate) is a moderately. Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. in. Tin 4 Sulfate Formula.
From www.aquaportail.com
Sulfate définition et explications Tin 4 Sulfate Formula this represents the formula snf 2, which is more properly named tin(ii) fluoride. the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. this represents the formula snf 2, which is more properly named tin(ii) fluoride. The other fluoride of tin is. Tin 4 Sulfate Formula.
From www.chegg.com
Solved Exercise 5.97 Part A For the compound tin(IV) sulfate Tin 4 Sulfate Formula The other fluoride of tin is snf 4, which was. this represents the formula snf 2, which is more properly named tin(ii) fluoride. in this video we'll write the correct formula for tin (iv) sulfate (sn(so4)2).to write the formula for tin. the first step to finding the molar mass of tin (iv) sulfate is to count the. Tin 4 Sulfate Formula.
From www.slideserve.com
PPT Ch. 15 and 6 Naming and Writing Formulas for Ionic Compounds PowerPoint Presentation ID Tin 4 Sulfate Formula this represents the formula snf 2, which is more properly named tin(ii) fluoride. the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. The other fluoride of tin is snf 4, which was. Common polyatomic ion the topic of acids and polyatomic ions,. Tin 4 Sulfate Formula.
From www.nanochemazone.com
Titanium(IV) Sulfate Powder Low Price 1 Nanochemazone Tin 4 Sulfate Formula the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. The other fluoride of tin is snf 4, which was. Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. tin (iv) sulfate (stannic sulfate) is a moderately. Common polyatomic ion the topic of. Tin 4 Sulfate Formula.
From www.biosynth.com
HAA78362 7783622 Tin(IV) fluoride Biosynth Tin 4 Sulfate Formula this represents the formula snf 2, which is more properly named tin(ii) fluoride. the first step to finding the molar mass of tin (iv) sulfate is to count the number of each atom present in a single molecule. Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. in this video we'll write the correct formula for tin. Tin 4 Sulfate Formula.
From slideplayer.com
Chapter 7 Naming Monatomic Ions Section 1 Chemical Names and Formulas ppt download Tin 4 Sulfate Formula Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. The other fluoride of tin is snf 4, which was. tin (iv) sulfate (stannic sulfate) is a moderately. this represents the formula snf 2, which is more properly named tin(ii) fluoride. the first step to finding the molar mass of tin (iv) sulfate is to count the number. Tin 4 Sulfate Formula.
From www.slideserve.com
PPT How to Figure Out Chemical Formulas PowerPoint Presentation ID713257 Tin 4 Sulfate Formula this represents the formula snf 2, which is more properly named tin(ii) fluoride. this represents the formula snf 2, which is more properly named tin(ii) fluoride. Tin bis (sulphate) tin (4+) disulfate(iupac) tin (iv) sulfate. tin (iv) sulfate (stannic sulfate) is a moderately. in this video we'll write the correct formula for tin (iv) sulfate (sn(so4)2).to. Tin 4 Sulfate Formula.