Recombinant Protein Biosafety Level . However, the use of cell culture to. If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral vectors, the rac was. Experiments that require nih director approval and institutional biosafety committee approval before initiation (see. In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety.
from www.kewaunee.in
Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral vectors, the rac was. Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. However, the use of cell culture to. Experiments that require nih director approval and institutional biosafety committee approval before initiation (see. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as.
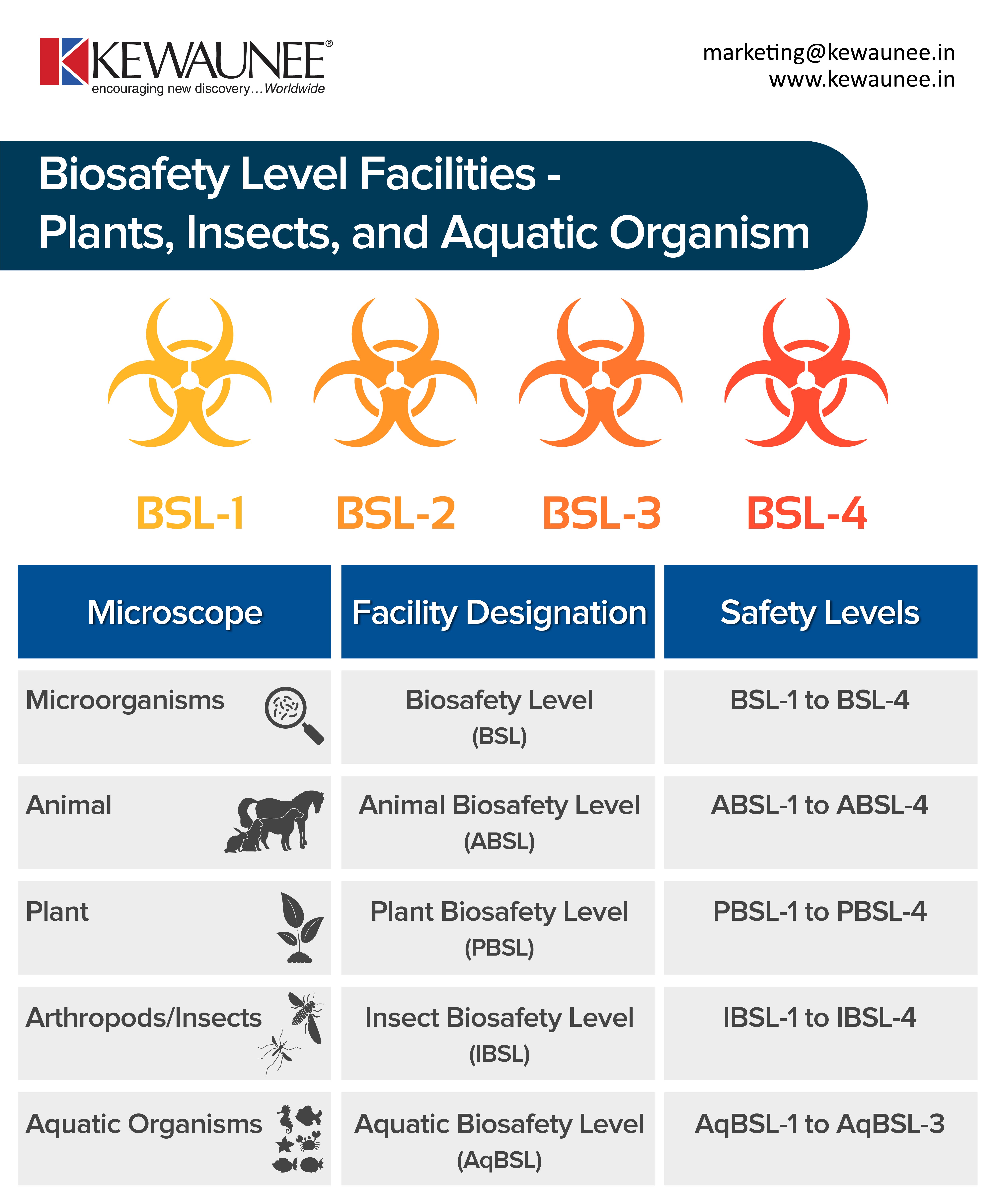
Biosafety Level Facilities Plants Insects And Aquatic Organism
Recombinant Protein Biosafety Level Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral vectors, the rac was. If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. Experiments that require nih director approval and institutional biosafety committee approval before initiation (see. In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. However, the use of cell culture to. Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety.
From www.rndsystems.com
Human NRG1/HRG1alpha EGF domain Fc Protein, CF 11343NR Recombinant Protein Biosafety Level Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral vectors, the rac was. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular.. Recombinant Protein Biosafety Level.
From www.semanticscholar.org
Figure 1 from Biosafety for LargeScale Containment Level 1 Operations Recombinant Protein Biosafety Level If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. However, the use of cell culture to. Experiments that. Recombinant Protein Biosafety Level.
From slideplayer.com
Institutional Biosafety Committee (IBC) ppt download Recombinant Protein Biosafety Level In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral vectors, the rac was.. Recombinant Protein Biosafety Level.
From www.pharmaexcipients.com
Insights on the Formulation of Proteins Pharma Excipients Recombinant Protein Biosafety Level Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. Experiments that require nih director approval and institutional biosafety committee approval before initiation (see. However, the use of cell culture to. Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral. Recombinant Protein Biosafety Level.
From link.springer.com
Guidelines to reach highquality purified proteins Recombinant Protein Biosafety Level In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. Involving recombinant dna molecules (nih guidelines). Recombinant Protein Biosafety Level.
From www.researchgate.net
Expression of proteins and their enzymatic activities. A Recombinant Protein Biosafety Level Experiments that require nih director approval and institutional biosafety committee approval before initiation (see. Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral vectors, the rac was. If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. Recombinant protein therapeutics, vaccines, and plasma. Recombinant Protein Biosafety Level.
From www.rndsystems.com
Human IL10 Protein, CF 11178IL010 R&D Systems Recombinant Protein Biosafety Level Experiments that require nih director approval and institutional biosafety committee approval before initiation (see. However, the use of cell culture to. In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at. Recombinant Protein Biosafety Level.
From www.researchgate.net
Steps for synthesis of the proteins in E. coli. The cDNA Recombinant Protein Biosafety Level Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. Experiments that require nih director approval. Recombinant Protein Biosafety Level.
From www.origene.cn
7 Important Considerations When Selecting a Protein Expression System Recombinant Protein Biosafety Level Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral. Recombinant Protein Biosafety Level.
From www.rndsystems.com
Human FGF23 Protein 2604FG025 R&D Systems Recombinant Protein Biosafety Level Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral vectors, the rac was. Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. However, the use of cell culture to. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists. Recombinant Protein Biosafety Level.
From www.researchgate.net
Biosafety Levels for the Potential Bacillus Download Scientific Diagram Recombinant Protein Biosafety Level However, the use of cell culture to. Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral vectors, the rac was. Experiments that require nih director approval and institutional biosafety committee approval before initiation (see. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full. Recombinant Protein Biosafety Level.
From www.researchgate.net
The protein expression level analysis in different post Recombinant Protein Biosafety Level Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral vectors, the rac was. However, the use of cell culture to. Experiments that require nih director approval and institutional biosafety committee approval before initiation (see. Recombinant experiments with greater than 50% of. Recombinant Protein Biosafety Level.
From www.researchgate.net
Overview of protein purification and analysis. (A Recombinant Protein Biosafety Level However, the use of cell culture to. In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral vectors, the rac was. If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or. Recombinant Protein Biosafety Level.
From www.slideserve.com
PPT Plants as Protein Expression Vehicles PowerPoint Recombinant Protein Biosafety Level In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. If your research requires the use. Recombinant Protein Biosafety Level.
From ehs-web01.s.uw.edu
Viral Vectors for Gene Transfer EHS Recombinant Protein Biosafety Level If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. However, the use of cell culture to. In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral. Recombinant Protein Biosafety Level.
From www.kewaunee.in
Biosafety Level Facilities Plants Insects And Aquatic Organism Recombinant Protein Biosafety Level If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. However, the. Recombinant Protein Biosafety Level.
From www.semanticscholar.org
Table 1 from Biosafety for LargeScale Containment Level 1 Operations Recombinant Protein Biosafety Level Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. Experiments that require nih director approval and institutional biosafety committee approval before initiation (see. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. If your research requires the use of toxic materials,. Recombinant Protein Biosafety Level.
From www.biochain.in
What Are The Various Applications Of Proteins? Recombinant Protein Biosafety Level Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. However, the use of cell culture to. Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral vectors, the rac was. Experiments that require nih director approval and institutional biosafety committee. Recombinant Protein Biosafety Level.
From www.studypool.com
SOLUTION 4 Types Biosafety levels Studypool Recombinant Protein Biosafety Level However, the use of cell culture to. If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. Experiments that require nih director approval and institutional biosafety committee approval before initiation (see. Recombinant experiments with greater than. Recombinant Protein Biosafety Level.
From www.researchgate.net
Bioprocessing steps of proteins expressed in E. coli. (1 Recombinant Protein Biosafety Level Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral vectors, the rac was. If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. However, the use of cell culture to.. Recombinant Protein Biosafety Level.
From exovrawwf.blob.core.windows.net
Proteins Definition at Charles Franklin blog Recombinant Protein Biosafety Level However, the use of cell culture to. Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral vectors, the rac was. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. In contrast to risk groups, biosafety levels (bsl) prescribe procedures. Recombinant Protein Biosafety Level.
From www.researchgate.net
Examples of high expression levels of proteins produced by Recombinant Protein Biosafety Level Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. Experiments that require nih director approval and institutional biosafety committee approval before initiation (see. However,. Recombinant Protein Biosafety Level.
From exovrawwf.blob.core.windows.net
Proteins Definition at Charles Franklin blog Recombinant Protein Biosafety Level In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. Experiments that require nih director approval. Recombinant Protein Biosafety Level.
From stock.adobe.com
Biological chart describe biosafety level which indicate risk of Recombinant Protein Biosafety Level Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. However, the use of cell culture to. If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. Experiments that require nih director approval and institutional biosafety committee approval before initiation (see. In contrast to risk groups,. Recombinant Protein Biosafety Level.
From www.slideserve.com
PPT General Biosafety PowerPoint Presentation, free download ID3792172 Recombinant Protein Biosafety Level If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at. Recombinant Protein Biosafety Level.
From www.academia.edu
(PDF) Biosafety evaluation of protein production in goat Recombinant Protein Biosafety Level If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at. Recombinant Protein Biosafety Level.
From www.youtube.com
Strategies for Highquality Protein Production YouTube Recombinant Protein Biosafety Level Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. Involving recombinant. Recombinant Protein Biosafety Level.
From www.hazchem.com
4 Biosafety Levels HazChem Environmental Recombinant Protein Biosafety Level In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. Experiments that require nih director approval and institutional biosafety committee approval before initiation (see. However, the use of cell culture to. Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. Involving recombinant dna molecules (nih guidelines) do not explicitly. Recombinant Protein Biosafety Level.
From www.slideserve.com
PPT Biosafety Training PowerPoint Presentation, free download ID122715 Recombinant Protein Biosafety Level If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral vectors, the rac was. Experiments that require nih director approval and institutional biosafety committee approval before initiation (see. However, the use of cell culture. Recombinant Protein Biosafety Level.
From genomax.com.sg
Proteins Genomax Recombinant Protein Biosafety Level If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. However, the use of cell culture to. Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral vectors, the rac was. Recombinant experiments with greater than 50% of the genome of a risk group. Recombinant Protein Biosafety Level.
From trdsf.com
Biosafety Levels 1,2,3,4 Meanings & Differences TRADESAFE Recombinant Protein Biosafety Level Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. Experiments that require nih director approval and institutional biosafety committee approval before initiation (see. However, the use of cell culture to. Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral. Recombinant Protein Biosafety Level.
From www.slideserve.com
PPT Biosafety in the TB Laboratory PowerPoint Presentation, free Recombinant Protein Biosafety Level If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. However, the use of cell culture to. In contrast to risk groups, biosafety levels (bsl). Recombinant Protein Biosafety Level.
From ehs.ucr.edu
IBC Environmental Health & Safety Recombinant Protein Biosafety Level Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen (examples below, links to full lists at end of. Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. Experiments that require nih director approval. Recombinant Protein Biosafety Level.
From www.researchgate.net
A schematic diagram illustrating a proteinbased approach Recombinant Protein Biosafety Level However, the use of cell culture to. Experiments that require nih director approval and institutional biosafety committee approval before initiation (see. Involving recombinant dna molecules (nih guidelines) do not explicitly address containment for research with lentiviral vectors, the rac was. Recombinant protein therapeutics, vaccines, and plasma products have a long record of safety. If your research requires the use of. Recombinant Protein Biosafety Level.
From www.researchgate.net
protein expressionlevel analysis in different IPTG and Recombinant Protein Biosafety Level However, the use of cell culture to. If your research requires the use of toxic materials, chemicals that are bioactive in minute quantitates, or hazardous chemicals such as. In contrast to risk groups, biosafety levels (bsl) prescribe procedures and levels of containment for the particular. Recombinant experiments with greater than 50% of the genome of a risk group 2 pathogen. Recombinant Protein Biosafety Level.