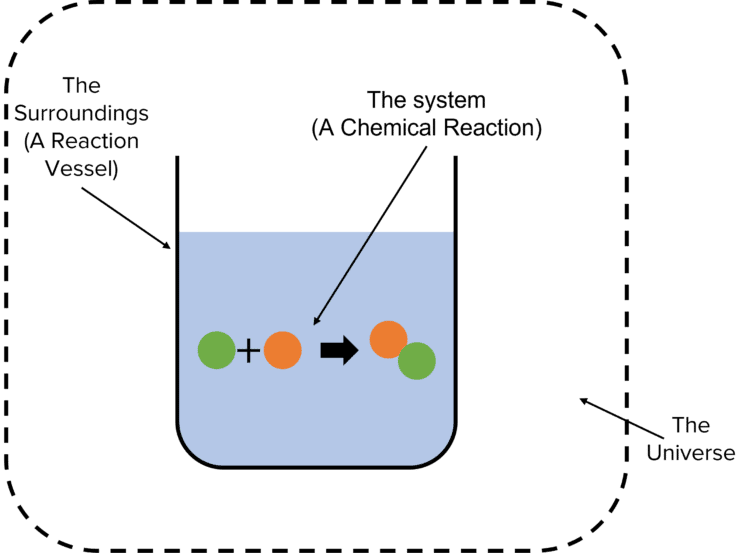
Endothermic Reaction System And Surroundings . That explains why we feel cold when inside a canopy of trees. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction causes the surroundings to cool down. Some examples of endothermic reactions are: Because heat is absorbed, endothermic. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. The reaction between ethanoic acid and sodium carbonate.
from mmerevise.co.uk
Some examples of endothermic reactions are: That explains why we feel cold when inside a canopy of trees. An endothermic reaction causes the surroundings to cool down. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Because heat is absorbed, endothermic.
Endothermic and Exothermic Reactions Revision MME
Endothermic Reaction System And Surroundings The reaction between ethanoic acid and sodium carbonate. Because heat is absorbed, endothermic. Some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. An endothermic reaction causes the surroundings to cool down. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. That explains why we feel cold when inside a canopy of trees. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings.
From slidetodoc.com
Endothermic and Exothermic Reactions System Vs Surrounding System Endothermic Reaction System And Surroundings A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Some examples of endothermic reactions are: An endothermic reaction causes the surroundings to cool down. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. An endothermic reaction is a chemical change in which the system absorbs thermal. Endothermic Reaction System And Surroundings.
From slideplayer.com
Thermochemistry Chemistry One B. ppt download Endothermic Reaction System And Surroundings A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its. Endothermic Reaction System And Surroundings.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation Endothermic Reaction System And Surroundings A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction causes the surroundings to cool down. That explains why we feel cold when inside a canopy of trees. Because heat is absorbed, endothermic. Some examples of endothermic reactions are: A chemical reaction or physical change is endothermic if heat. Endothermic Reaction System And Surroundings.
From letstalkscience.ca
The Cold Pack A Chilly Example of an Endothermic Reaction Let's Talk Endothermic Reaction System And Surroundings Some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. An endothermic reaction causes the surroundings to cool down. That explains why we feel cold when inside a canopy of trees. In the course of an endothermic process, the system gains heat. Endothermic Reaction System And Surroundings.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reaction System And Surroundings A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. The reaction between ethanoic acid and sodium carbonate. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Some examples. Endothermic Reaction System And Surroundings.
From slideplayer.com
Thermochemistry Chemistry One B. ppt download Endothermic Reaction System And Surroundings In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. Because heat is absorbed, endothermic. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Some. Endothermic Reaction System And Surroundings.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction System And Surroundings A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Because heat is absorbed, endothermic. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. That explains why we feel cold when inside a canopy of trees.. Endothermic Reaction System And Surroundings.
From www.savemyexams.com
Energy Diagrams College Board AP Chemistry Revision Notes 2022 Endothermic Reaction System And Surroundings A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction causes the surroundings to cool down. That. Endothermic Reaction System And Surroundings.
From slidetodoc.com
Exothermic and Endothermic reactions Learning Objectives Explain heat Endothermic Reaction System And Surroundings A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Because heat is absorbed, endothermic. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction causes. Endothermic Reaction System And Surroundings.
From www.vecteezy.com
Enthalpy Endothermic versus Endothermic biochemistry vector Endothermic Reaction System And Surroundings An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. The reaction between ethanoic acid and sodium carbonate. A chemical reaction or physical change is endothermic if. Endothermic Reaction System And Surroundings.
From slideplayer.com
Chapter Six THERMOCHEMISTRY. ppt download Endothermic Reaction System And Surroundings In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. That explains why we feel cold when inside a canopy of trees. An. Endothermic Reaction System And Surroundings.
From slidetodoc.com
Endothermic and Exothermic Reactions System Vs Surrounding System Endothermic Reaction System And Surroundings In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Some examples of endothermic reactions are:. Endothermic Reaction System And Surroundings.
From www.slideserve.com
PPT Chapter 17 “Thermochemistry” PowerPoint Presentation, free Endothermic Reaction System And Surroundings Because heat is absorbed, endothermic. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction causes the surroundings to cool down. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction is a chemical reaction that absorbs thermal. Endothermic Reaction System And Surroundings.
From slideplayer.com
Thermochemistry Chemistry One B. ppt download Endothermic Reaction System And Surroundings The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Because heat is absorbed, endothermic. An endothermic reaction is. Endothermic Reaction System And Surroundings.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID4499204 Endothermic Reaction System And Surroundings An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. That explains why we feel cold when inside a canopy of trees. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. In the course of an. Endothermic Reaction System And Surroundings.
From question.pandai.org
Endothermic and exothermic reactions Endothermic Reaction System And Surroundings The reaction between ethanoic acid and sodium carbonate. An endothermic reaction causes the surroundings to cool down. That explains why we feel cold when inside a canopy of trees. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. In the course of an endothermic process, the system gains heat from the. Endothermic Reaction System And Surroundings.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction System And Surroundings That explains why we feel cold when inside a canopy of trees. An endothermic reaction causes the surroundings to cool down. The reaction between ethanoic acid and sodium carbonate. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Because heat is absorbed, endothermic. An endothermic reaction is a chemical change in. Endothermic Reaction System And Surroundings.
From slideplayer.com
Rate of Chemical Reactions Unit 3 AOS 2 ppt download Endothermic Reaction System And Surroundings The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. Because heat is absorbed, endothermic. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Some examples of endothermic reactions are: An endothermic. Endothermic Reaction System And Surroundings.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Endothermic Reaction System And Surroundings The reaction between ethanoic acid and sodium carbonate. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Some examples of endothermic reactions are: Because heat is absorbed, endothermic. That explains why we feel cold when inside a canopy of trees. In the course of an endothermic process, the system gains heat. Endothermic Reaction System And Surroundings.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction System And Surroundings An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction causes the surroundings to cool down. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is a chemical change in which the system. Endothermic Reaction System And Surroundings.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction System And Surroundings A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Because heat is absorbed, endothermic. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of. Endothermic Reaction System And Surroundings.
From slideplayer.com
Thermochemistry Chemistry One B. ppt download Endothermic Reaction System And Surroundings An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Some examples of endothermic reactions are: That explains why we feel cold when inside a canopy of trees. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. A chemical reaction or physical change. Endothermic Reaction System And Surroundings.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction System And Surroundings An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. That explains why we feel cold when inside a canopy of trees. Some examples of endothermic reactions are: A chemical reaction or physical change is endothermic if heat is absorbed by the system from the. Endothermic Reaction System And Surroundings.
From www.savemyexams.com
Exothermic & Endothermic Edexcel IGCSE Chemistry Revision Notes 2019 Endothermic Reaction System And Surroundings An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Some examples of endothermic reactions are: An endothermic reaction causes the surroundings to cool down. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. Because heat is absorbed, endothermic. That. Endothermic Reaction System And Surroundings.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Endothermic Reaction System And Surroundings An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. In the course of an endothermic process, the system gains heat from the surroundings and so the. Endothermic Reaction System And Surroundings.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction System And Surroundings Because heat is absorbed, endothermic. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Some examples of endothermic reactions are: An endothermic reaction causes the surroundings. Endothermic Reaction System And Surroundings.
From general.chemistrysteps.com
Entropy Changes in the Surroundings Chemistry Steps Endothermic Reaction System And Surroundings The reaction between ethanoic acid and sodium carbonate. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. Because heat is absorbed, endothermic. That explains why we feel cold when. Endothermic Reaction System And Surroundings.
From slideplayer.com
Thermochemistry Chapter ppt download Endothermic Reaction System And Surroundings A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. An endothermic reaction causes the surroundings to cool down. That explains why we feel cold when inside. Endothermic Reaction System And Surroundings.
From www.expii.com
Endothermic and Exothermic Reactions — Overview & Comparison Expii Endothermic Reaction System And Surroundings An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. An endothermic reaction causes the surroundings to cool down. An endothermic reaction is a chemical change in which the system absorbs thermal energy from. Endothermic Reaction System And Surroundings.
From general.chemistrysteps.com
What is Enthalpy Chemistry Steps Endothermic Reaction System And Surroundings A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Because heat is absorbed, endothermic. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Some examples of endothermic reactions. Endothermic Reaction System And Surroundings.
From general.chemistrysteps.com
Endothermic and Exothermic Processes Chemistry Steps Endothermic Reaction System And Surroundings An endothermic reaction causes the surroundings to cool down. Some examples of endothermic reactions are: The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. In the course of an endothermic process, the system gains heat from. Endothermic Reaction System And Surroundings.
From www.sexiezpix.com
What Are Endothermic Reactions With Examples Video SexiezPix Porn Endothermic Reaction System And Surroundings A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Some examples of endothermic reactions are: Because heat is absorbed, endothermic. The reaction between ethanoic acid and sodium carbonate. That explains why we feel cold when inside a canopy of trees. In the course of an endothermic process, the system gains heat. Endothermic Reaction System And Surroundings.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction System And Surroundings Because heat is absorbed, endothermic. That explains why we feel cold when inside a canopy of trees. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. A chemical reaction or physical change is endothermic if heat is. Endothermic Reaction System And Surroundings.
From www.chemistrystudent.com
Enthalpy Changes (ALevel) ChemistryStudent Endothermic Reaction System And Surroundings A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. The reaction between ethanoic acid and sodium carbonate. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the. Endothermic Reaction System And Surroundings.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID4499939 Endothermic Reaction System And Surroundings The reaction between ethanoic acid and sodium carbonate. Some examples of endothermic reactions are: In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. That explains why we feel cold when inside a canopy of trees. An endothermic reaction causes the surroundings to cool down. A chemical reaction. Endothermic Reaction System And Surroundings.