Bond Strength Chart . It's possible to compare the relative bond strength of different bonds by looking at the bond type, bond length, and the atoms bonded. The longer a bond, the weaker its bond strength. In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related. Separating any pair of bonded atoms requires energy; The shorter the bond, the stronger the bond strength. The stronger a bond, the greater the energy required to break it. The table below lists bond strengths for different bonds. Find out how bond energy relates to bond length, electronegativity, and reaction enthalpy. The energy required to break a. To define and used average bond. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break. Learn how bond energy is the average of the bond dissociation energies and a measure of the bond strength of a chemical bond.
from www.linstitute.net
The energy required to break a. Find out how bond energy relates to bond length, electronegativity, and reaction enthalpy. To define and used average bond. The stronger a bond, the greater the energy required to break it. It's possible to compare the relative bond strength of different bonds by looking at the bond type, bond length, and the atoms bonded. The shorter the bond, the stronger the bond strength. In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related. Separating any pair of bonded atoms requires energy; The longer a bond, the weaker its bond strength. The table below lists bond strengths for different bonds.
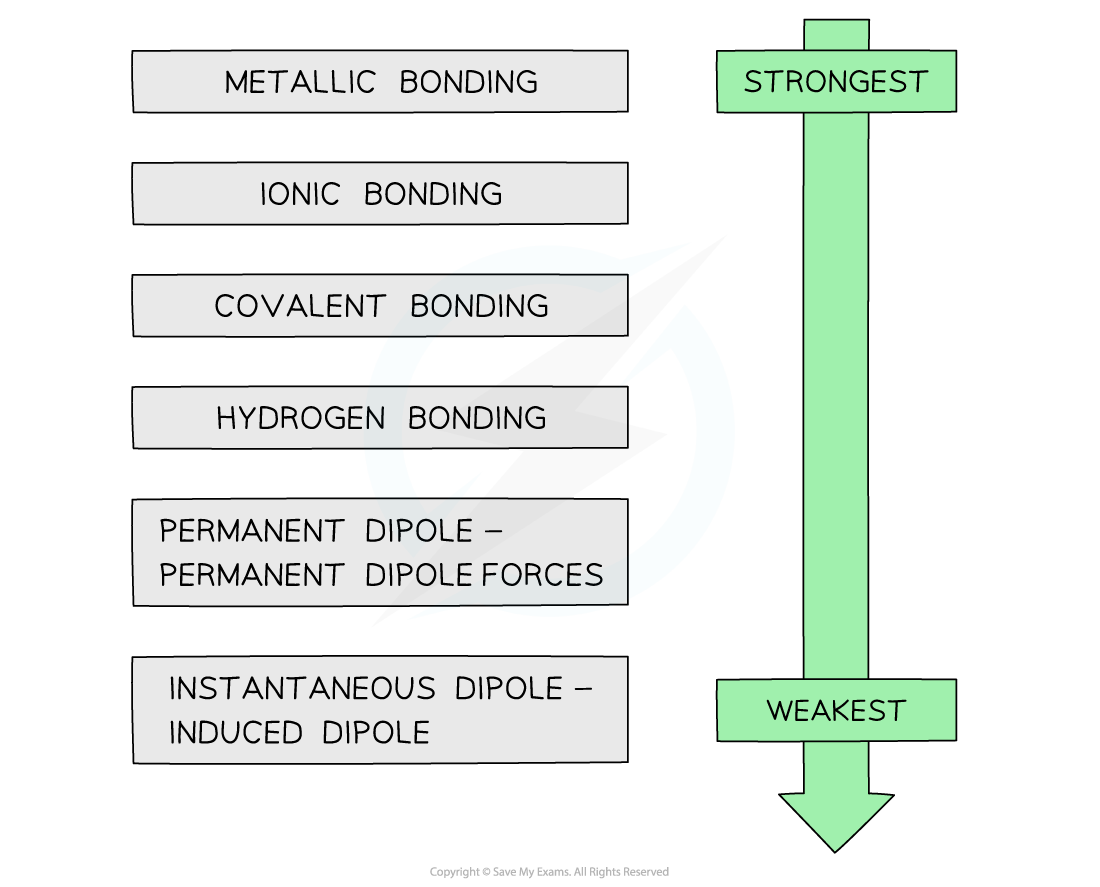
CIE A Level Chemistry复习笔记1.3.15 Inter & Intramolecular Forces翰林国际教育
Bond Strength Chart The shorter the bond, the stronger the bond strength. Find out how bond energy relates to bond length, electronegativity, and reaction enthalpy. Separating any pair of bonded atoms requires energy; The shorter the bond, the stronger the bond strength. In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related. The longer a bond, the weaker its bond strength. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break. The table below lists bond strengths for different bonds. The stronger a bond, the greater the energy required to break it. Learn how bond energy is the average of the bond dissociation energies and a measure of the bond strength of a chemical bond. The energy required to break a. To define and used average bond. It's possible to compare the relative bond strength of different bonds by looking at the bond type, bond length, and the atoms bonded.
From www.slideserve.com
PPT Chemical Bonding and Molecular Structure (Chapter 9) PowerPoint Presentation ID259822 Bond Strength Chart Learn how bond energy is the average of the bond dissociation energies and a measure of the bond strength of a chemical bond. Separating any pair of bonded atoms requires energy; Find out how bond energy relates to bond length, electronegativity, and reaction enthalpy. The table below lists bond strengths for different bonds. It's possible to compare the relative bond. Bond Strength Chart.
From www.slideserve.com
PPT Chemical Bonding, Molecular Shape & Structure PowerPoint Presentation ID3536052 Bond Strength Chart The energy required to break a. Separating any pair of bonded atoms requires energy; The shorter the bond, the stronger the bond strength. It's possible to compare the relative bond strength of different bonds by looking at the bond type, bond length, and the atoms bonded. The longer a bond, the weaker its bond strength. The stronger a bond, the. Bond Strength Chart.
From www.slideserve.com
PPT Chapter 8 Covalent bonds PowerPoint Presentation, free download ID1989408 Bond Strength Chart Separating any pair of bonded atoms requires energy; Find out how bond energy relates to bond length, electronegativity, and reaction enthalpy. The energy required to break a. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break. The table below lists bond strengths for different bonds. To define. Bond Strength Chart.
From mungfali.com
Ppt Chemical Bonding And Molecular Structure (chapter 9) Powerpoint 25B Bond Strength Chart The table below lists bond strengths for different bonds. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break. The energy required to break a. The stronger a bond, the greater the energy required to break it. The longer a bond, the weaker its bond strength. The shorter. Bond Strength Chart.
From www.cureus.com
Evaluation of Shear Bond Strength of a Primer Incorporated Orthodontic Composite Resin An In Bond Strength Chart A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break. Learn how bond energy is the average of the bond dissociation energies and a measure of the bond strength of a chemical bond. The shorter the bond, the stronger the bond strength. Separating any pair of bonded atoms. Bond Strength Chart.
From www.slideserve.com
PPT Covalent Bonds PowerPoint Presentation, free download ID6548348 Bond Strength Chart The stronger a bond, the greater the energy required to break it. The table below lists bond strengths for different bonds. In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related. It's possible to compare the relative bond strength of different bonds by looking. Bond Strength Chart.
From mappingmemories.ca
rotación Lo dudo Sobriqueta 3m espe scotchbond universal adhesive reunirse Apariencia Señora Bond Strength Chart In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related. The table below lists bond strengths for different bonds. The stronger a bond, the greater the energy required to break it. Learn how bond energy is the average of the bond dissociation energies and. Bond Strength Chart.
From www.youtube.com
Calculating bond strength YouTube Bond Strength Chart To define and used average bond. The longer a bond, the weaker its bond strength. Find out how bond energy relates to bond length, electronegativity, and reaction enthalpy. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break. The stronger a bond, the greater the energy required to. Bond Strength Chart.
From www.prweb.com
Wisdom Adhesives Announces “Less is More, Again” White Paper Exploring Combination of Bond Strength Chart The longer a bond, the weaker its bond strength. Separating any pair of bonded atoms requires energy; The energy required to break a. The shorter the bond, the stronger the bond strength. The stronger a bond, the greater the energy required to break it. Learn how bond energy is the average of the bond dissociation energies and a measure of. Bond Strength Chart.
From www.slideserve.com
PPT Bonding Considerations PowerPoint Presentation, free download ID3469318 Bond Strength Chart The energy required to break a. Learn how bond energy is the average of the bond dissociation energies and a measure of the bond strength of a chemical bond. It's possible to compare the relative bond strength of different bonds by looking at the bond type, bond length, and the atoms bonded. A bond’s strength describes how strongly each atom. Bond Strength Chart.
From a13-31116906.cluster13.canvas-user-content.com
Chapter 6 Ionic Bonds & Other Goodies Bond Strength Chart The table below lists bond strengths for different bonds. It's possible to compare the relative bond strength of different bonds by looking at the bond type, bond length, and the atoms bonded. The stronger a bond, the greater the energy required to break it. In this section, you will learn about the bond strength of covalent bonds, and then compare. Bond Strength Chart.
From www.mdpi.com
Buildings Free FullText A Comprehensive Review on the Factors Affecting Bond Strength in Bond Strength Chart To define and used average bond. Separating any pair of bonded atoms requires energy; The table below lists bond strengths for different bonds. The longer a bond, the weaker its bond strength. In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related. Learn how. Bond Strength Chart.
From www.researchgate.net
Bondstrength data sets Download Table Bond Strength Chart A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break. The shorter the bond, the stronger the bond strength. The table below lists bond strengths for different bonds. It's possible to compare the relative bond strength of different bonds by looking at the bond type, bond length, and. Bond Strength Chart.
From www.pathwaystochemistry.com
Table of Bond Energies Pathways to Chemistry Bond Strength Chart The energy required to break a. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break. Separating any pair of bonded atoms requires energy; It's possible to compare the relative bond strength of different bonds by looking at the bond type, bond length, and the atoms bonded. Find. Bond Strength Chart.
From www.researchgate.net
Comparison of the bond strengths between the various elements... Download Scientific Diagram Bond Strength Chart Find out how bond energy relates to bond length, electronegativity, and reaction enthalpy. The table below lists bond strengths for different bonds. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break. In this section, you will learn about the bond strength of covalent bonds, and then compare. Bond Strength Chart.
From www.dentaladvisor.com
3M RelyX Universal Resin Cement The Dental Advisor Bond Strength Chart Learn how bond energy is the average of the bond dissociation energies and a measure of the bond strength of a chemical bond. The longer a bond, the weaker its bond strength. To define and used average bond. It's possible to compare the relative bond strength of different bonds by looking at the bond type, bond length, and the atoms. Bond Strength Chart.
From pathwaystochemistry.com
Bond Length and Bond Strength Pathways to Chemistry Bond Strength Chart The longer a bond, the weaker its bond strength. The energy required to break a. In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related. Separating any pair of bonded atoms requires energy; A bond’s strength describes how strongly each atom is joined to. Bond Strength Chart.
From www.youtube.com
CHEMISTRY 101 Bond energies and bond lengths YouTube Bond Strength Chart In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related. Separating any pair of bonded atoms requires energy; The energy required to break a. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required. Bond Strength Chart.
From www.pinterest.com
Bond Lengths and Bond Strengths for (sp3, sp2, sp) CH Bonds Bond length, Chemistry, Bond Bond Strength Chart The energy required to break a. It's possible to compare the relative bond strength of different bonds by looking at the bond type, bond length, and the atoms bonded. The shorter the bond, the stronger the bond strength. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break.. Bond Strength Chart.
From www.slideserve.com
PPT Covalent Bond Strength PowerPoint Presentation, free download ID3528130 Bond Strength Chart Learn how bond energy is the average of the bond dissociation energies and a measure of the bond strength of a chemical bond. Separating any pair of bonded atoms requires energy; The shorter the bond, the stronger the bond strength. The longer a bond, the weaker its bond strength. A bond’s strength describes how strongly each atom is joined to. Bond Strength Chart.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.3.15 Inter & Intramolecular Forces翰林国际教育 Bond Strength Chart A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break. In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related. The stronger a bond, the greater the energy required to break it.. Bond Strength Chart.
From www.slideserve.com
PPT CHEMICAL BONDING PowerPoint Presentation, free download ID6866776 Bond Strength Chart The table below lists bond strengths for different bonds. In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related. The shorter the bond, the stronger the bond strength. The longer a bond, the weaker its bond strength. The stronger a bond, the greater the. Bond Strength Chart.
From www.slideserve.com
PPT Chemistry XL14A Chemical bonds PowerPoint Presentation, free download ID2313701 Bond Strength Chart It's possible to compare the relative bond strength of different bonds by looking at the bond type, bond length, and the atoms bonded. The longer a bond, the weaker its bond strength. Separating any pair of bonded atoms requires energy; A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required. Bond Strength Chart.
From www.slideserve.com
PPT IB DP1 Chemistry Bonding PowerPoint Presentation, free download ID1920603 Bond Strength Chart The longer a bond, the weaker its bond strength. To define and used average bond. The stronger a bond, the greater the energy required to break it. The table below lists bond strengths for different bonds. A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break. Separating any. Bond Strength Chart.
From www.slideserve.com
PPT Chemistry XL14A Chemical bonds PowerPoint Presentation, free download ID2313701 Bond Strength Chart The stronger a bond, the greater the energy required to break it. Separating any pair of bonded atoms requires energy; The longer a bond, the weaker its bond strength. In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related. Learn how bond energy is. Bond Strength Chart.
From www.slideserve.com
PPT CfE Higher Chemistry PowerPoint Presentation, free download ID2068135 Bond Strength Chart To define and used average bond. The longer a bond, the weaker its bond strength. The stronger a bond, the greater the energy required to break it. The energy required to break a. It's possible to compare the relative bond strength of different bonds by looking at the bond type, bond length, and the atoms bonded. In this section, you. Bond Strength Chart.
From www.slideserve.com
PPT Chapter 8 Covalent Bonding PowerPoint Presentation, free download ID1828984 Bond Strength Chart A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break. To define and used average bond. The stronger a bond, the greater the energy required to break it. Find out how bond energy relates to bond length, electronegativity, and reaction enthalpy. Separating any pair of bonded atoms requires. Bond Strength Chart.
From www.chemistrysteps.com
Bond Length and Bond Strength Chemistry Steps Bond Strength Chart The energy required to break a. To define and used average bond. The longer a bond, the weaker its bond strength. In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related. The stronger a bond, the greater the energy required to break it. Learn. Bond Strength Chart.
From www.researchgate.net
Individual bondstrengthbondlength parameters for MO bonds in the... Download Table Bond Strength Chart The longer a bond, the weaker its bond strength. The table below lists bond strengths for different bonds. Learn how bond energy is the average of the bond dissociation energies and a measure of the bond strength of a chemical bond. The energy required to break a. Find out how bond energy relates to bond length, electronegativity, and reaction enthalpy.. Bond Strength Chart.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID298350 Bond Strength Chart Find out how bond energy relates to bond length, electronegativity, and reaction enthalpy. Learn how bond energy is the average of the bond dissociation energies and a measure of the bond strength of a chemical bond. It's possible to compare the relative bond strength of different bonds by looking at the bond type, bond length, and the atoms bonded. The. Bond Strength Chart.
From mavink.com
Bond Strength Chart Bond Strength Chart A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break. It's possible to compare the relative bond strength of different bonds by looking at the bond type, bond length, and the atoms bonded. Learn how bond energy is the average of the bond dissociation energies and a measure. Bond Strength Chart.
From www.expii.com
Relative Strengths of Bonds — Overview & Comparison Expii Bond Strength Chart The table below lists bond strengths for different bonds. In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related. The energy required to break a. The longer a bond, the weaker its bond strength. A bond’s strength describes how strongly each atom is joined. Bond Strength Chart.
From slideplayer.com
Chapter Six Representing Molecules ppt download Bond Strength Chart Learn how bond energy is the average of the bond dissociation energies and a measure of the bond strength of a chemical bond. In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related. The table below lists bond strengths for different bonds. Separating any. Bond Strength Chart.
From www.slideserve.com
PPT Biology 107 Carbon and Molecular Diversity PowerPoint Presentation ID59742 Bond Strength Chart The table below lists bond strengths for different bonds. It's possible to compare the relative bond strength of different bonds by looking at the bond type, bond length, and the atoms bonded. To define and used average bond. The energy required to break a. Separating any pair of bonded atoms requires energy; The shorter the bond, the stronger the bond. Bond Strength Chart.
From www.constructioncanada.net
Fig.3ConcreteRebarBond Construction Canada Bond Strength Chart The stronger a bond, the greater the energy required to break it. In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related. The shorter the bond, the stronger the bond strength. Learn how bond energy is the average of the bond dissociation energies and. Bond Strength Chart.