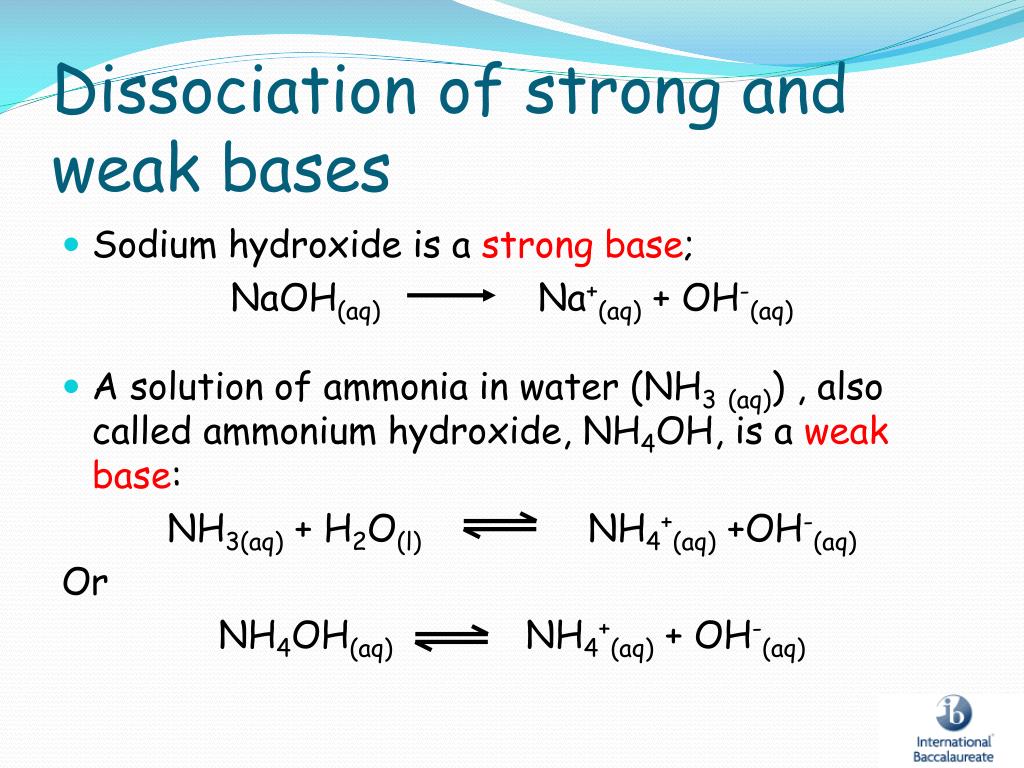
What Does Weak Base Mean . A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one which doesn't convert fully into hydroxide ions in solution. When a weak base reacts with water, the position of. Here is the definition of a weak base as the term is. A weak base is one that does not completely dissociate into its ions in water.
from www.slideserve.com
When a weak base reacts with water, the position of. A weak base is one which doesn't convert fully into hydroxide ions in solution. Here is the definition of a weak base as the term is. A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one that does not completely dissociate into its ions in water.
PPT Acids and Bases PowerPoint Presentation, free download ID6973259
What Does Weak Base Mean A weak base is a base that is partially dissociated in an aqueous solution. Here is the definition of a weak base as the term is. A weak base is one which doesn't convert fully into hydroxide ions in solution. A weak base is one that does not completely dissociate into its ions in water. A weak base is a base that is partially dissociated in an aqueous solution. When a weak base reacts with water, the position of.
From www.slideserve.com
PPT Equilibrium Part 2 Chemistry 2 PowerPoint Presentation, free download ID5413446 What Does Weak Base Mean A weak base is one which doesn't convert fully into hydroxide ions in solution. Here is the definition of a weak base as the term is. A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one that does not completely dissociate into its ions in water. When a weak base reacts. What Does Weak Base Mean.
From www.slideserve.com
PPT Weak Acids Weak Bases PowerPoint Presentation, free download ID3571409 What Does Weak Base Mean A weak base is one which doesn't convert fully into hydroxide ions in solution. Here is the definition of a weak base as the term is. A weak base is one that does not completely dissociate into its ions in water. A weak base is a base that is partially dissociated in an aqueous solution. When a weak base reacts. What Does Weak Base Mean.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions Part I PowerPoint Presentation What Does Weak Base Mean A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one which doesn't convert fully into hydroxide ions in solution. Here is the definition of a weak base as the term is. A weak base is one that does not completely dissociate into its ions in water. When a weak base reacts. What Does Weak Base Mean.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts What Does Weak Base Mean Here is the definition of a weak base as the term is. A weak base is one which doesn't convert fully into hydroxide ions in solution. A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one that does not completely dissociate into its ions in water. When a weak base reacts. What Does Weak Base Mean.
From www.youtube.com
Strong vs. Weak Bases What's the difference? How do they dissociate? The 8 Strong Bases YouTube What Does Weak Base Mean Here is the definition of a weak base as the term is. When a weak base reacts with water, the position of. A weak base is one which doesn't convert fully into hydroxide ions in solution. A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one that does not completely dissociate. What Does Weak Base Mean.
From www.sliderbase.com
Buffers Presentation Chemistry What Does Weak Base Mean A weak base is one which doesn't convert fully into hydroxide ions in solution. A weak base is one that does not completely dissociate into its ions in water. A weak base is a base that is partially dissociated in an aqueous solution. When a weak base reacts with water, the position of. Here is the definition of a weak. What Does Weak Base Mean.
From facts.net
12 Mindblowing Facts About Basicity Constants Of Weak Bases What Does Weak Base Mean A weak base is one that does not completely dissociate into its ions in water. When a weak base reacts with water, the position of. A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one which doesn't convert fully into hydroxide ions in solution. Here is the definition of a weak. What Does Weak Base Mean.
From www.youtube.com
Strong & Weak Acids and Bases YouTube What Does Weak Base Mean Here is the definition of a weak base as the term is. A weak base is one which doesn't convert fully into hydroxide ions in solution. When a weak base reacts with water, the position of. A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one that does not completely dissociate. What Does Weak Base Mean.
From www.teachoo.com
Difference between Strong and Weak Base with Examples [in Table] What Does Weak Base Mean Here is the definition of a weak base as the term is. When a weak base reacts with water, the position of. A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one that does not completely dissociate into its ions in water. A weak base is one which doesn't convert fully. What Does Weak Base Mean.
From www.slideserve.com
PPT Unit 3 PowerPoint Presentation, free download ID2956486 What Does Weak Base Mean A weak base is one which doesn't convert fully into hydroxide ions in solution. When a weak base reacts with water, the position of. A weak base is one that does not completely dissociate into its ions in water. A weak base is a base that is partially dissociated in an aqueous solution. Here is the definition of a weak. What Does Weak Base Mean.
From slidesharetrick.blogspot.com
Difference Between Strong And Weak Acids Bases slidesharetrick What Does Weak Base Mean Here is the definition of a weak base as the term is. When a weak base reacts with water, the position of. A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one that does not completely dissociate into its ions in water. A weak base is one which doesn't convert fully. What Does Weak Base Mean.
From www.ck12.org
Weak Acids and Bases Overview ( Video ) Chemistry CK12 Foundation What Does Weak Base Mean Here is the definition of a weak base as the term is. A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one which doesn't convert fully into hydroxide ions in solution. When a weak base reacts with water, the position of. A weak base is one that does not completely dissociate. What Does Weak Base Mean.
From www.slideserve.com
PPT Strong and Weak Acids and Bases PowerPoint Presentation, free download ID2090242 What Does Weak Base Mean A weak base is one that does not completely dissociate into its ions in water. A weak base is a base that is partially dissociated in an aqueous solution. Here is the definition of a weak base as the term is. A weak base is one which doesn't convert fully into hydroxide ions in solution. When a weak base reacts. What Does Weak Base Mean.
From pediaa.com
Difference Between Strong and Weak Bases Definition, Properties, Reactions, Examples What Does Weak Base Mean When a weak base reacts with water, the position of. A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one which doesn't convert fully into hydroxide ions in solution. A weak base is one that does not completely dissociate into its ions in water. Here is the definition of a weak. What Does Weak Base Mean.
From www.slideserve.com
PPT Weak Acids and Bases PowerPoint Presentation, free download ID2220428 What Does Weak Base Mean When a weak base reacts with water, the position of. A weak base is one that does not completely dissociate into its ions in water. Here is the definition of a weak base as the term is. A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one which doesn't convert fully. What Does Weak Base Mean.
From www.sliderbase.com
Buffers Presentation Chemistry What Does Weak Base Mean A weak base is one which doesn't convert fully into hydroxide ions in solution. When a weak base reacts with water, the position of. A weak base is one that does not completely dissociate into its ions in water. A weak base is a base that is partially dissociated in an aqueous solution. Here is the definition of a weak. What Does Weak Base Mean.
From www.difference.wiki
Weak Base vs. Strong Base What’s the Difference? What Does Weak Base Mean Here is the definition of a weak base as the term is. A weak base is a base that is partially dissociated in an aqueous solution. When a weak base reacts with water, the position of. A weak base is one that does not completely dissociate into its ions in water. A weak base is one which doesn't convert fully. What Does Weak Base Mean.
From www.slideserve.com
PPT Unit 9 Notes PowerPoint Presentation, free download ID5460662 What Does Weak Base Mean A weak base is one which doesn't convert fully into hydroxide ions in solution. A weak base is a base that is partially dissociated in an aqueous solution. When a weak base reacts with water, the position of. A weak base is one that does not completely dissociate into its ions in water. Here is the definition of a weak. What Does Weak Base Mean.
From www.youtube.com
Weak acidweak base reactions Acids and bases AP Chemistry Khan Academy YouTube What Does Weak Base Mean A weak base is one which doesn't convert fully into hydroxide ions in solution. When a weak base reacts with water, the position of. A weak base is a base that is partially dissociated in an aqueous solution. Here is the definition of a weak base as the term is. A weak base is one that does not completely dissociate. What Does Weak Base Mean.
From www.expii.com
The pH of Weak Acid and Base Solutions — Examples Expii What Does Weak Base Mean Here is the definition of a weak base as the term is. A weak base is one which doesn't convert fully into hydroxide ions in solution. A weak base is one that does not completely dissociate into its ions in water. A weak base is a base that is partially dissociated in an aqueous solution. When a weak base reacts. What Does Weak Base Mean.
From www.teachoo.com
Examples of Weak Base (5 Examples with Images) Teachoo Chemistry What Does Weak Base Mean When a weak base reacts with water, the position of. A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one which doesn't convert fully into hydroxide ions in solution. Here is the definition of a weak base as the term is. A weak base is one that does not completely dissociate. What Does Weak Base Mean.
From www.slideserve.com
PPT Weak Bases PowerPoint Presentation, free download ID2838064 What Does Weak Base Mean A weak base is one which doesn't convert fully into hydroxide ions in solution. A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one that does not completely dissociate into its ions in water. Here is the definition of a weak base as the term is. When a weak base reacts. What Does Weak Base Mean.
From www.slideserve.com
PPT Acids and Bases Introduction PowerPoint Presentation, free download ID5408766 What Does Weak Base Mean A weak base is a base that is partially dissociated in an aqueous solution. When a weak base reacts with water, the position of. Here is the definition of a weak base as the term is. A weak base is one which doesn't convert fully into hydroxide ions in solution. A weak base is one that does not completely dissociate. What Does Weak Base Mean.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download ID3938103 What Does Weak Base Mean A weak base is one which doesn't convert fully into hydroxide ions in solution. Here is the definition of a weak base as the term is. A weak base is one that does not completely dissociate into its ions in water. When a weak base reacts with water, the position of. A weak base is a base that is partially. What Does Weak Base Mean.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID291336 What Does Weak Base Mean A weak base is one which doesn't convert fully into hydroxide ions in solution. A weak base is a base that is partially dissociated in an aqueous solution. When a weak base reacts with water, the position of. A weak base is one that does not completely dissociate into its ions in water. Here is the definition of a weak. What Does Weak Base Mean.
From layton-blogcantrell.blogspot.com
Weak Base Examples What Does Weak Base Mean Here is the definition of a weak base as the term is. A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one which doesn't convert fully into hydroxide ions in solution. When a weak base reacts with water, the position of. A weak base is one that does not completely dissociate. What Does Weak Base Mean.
From www.slideserve.com
PPT Weak Acids Weak Bases PowerPoint Presentation, free download ID3571409 What Does Weak Base Mean A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one that does not completely dissociate into its ions in water. Here is the definition of a weak base as the term is. A weak base is one which doesn't convert fully into hydroxide ions in solution. When a weak base reacts. What Does Weak Base Mean.
From www.askdifference.com
Weak Base vs. Strong Base — What’s the Difference? What Does Weak Base Mean Here is the definition of a weak base as the term is. A weak base is one which doesn't convert fully into hydroxide ions in solution. When a weak base reacts with water, the position of. A weak base is one that does not completely dissociate into its ions in water. A weak base is a base that is partially. What Does Weak Base Mean.
From www.slideserve.com
PPT Weak Acids Weak Bases PowerPoint Presentation, free download ID3571409 What Does Weak Base Mean When a weak base reacts with water, the position of. A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one that does not completely dissociate into its ions in water. Here is the definition of a weak base as the term is. A weak base is one which doesn't convert fully. What Does Weak Base Mean.
From www.youtube.com
Weak Base Calculations in Chemistry YouTube What Does Weak Base Mean Here is the definition of a weak base as the term is. When a weak base reacts with water, the position of. A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one that does not completely dissociate into its ions in water. A weak base is one which doesn't convert fully. What Does Weak Base Mean.
From shiken.ai
Weak Acids and Bases What Does Weak Base Mean A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one that does not completely dissociate into its ions in water. When a weak base reacts with water, the position of. Here is the definition of a weak base as the term is. A weak base is one which doesn't convert fully. What Does Weak Base Mean.
From quizdbcornwallis.z21.web.core.windows.net
What Makes A Base Weak What Does Weak Base Mean A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one that does not completely dissociate into its ions in water. A weak base is one which doesn't convert fully into hydroxide ions in solution. Here is the definition of a weak base as the term is. When a weak base reacts. What Does Weak Base Mean.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation, free download ID2958858 What Does Weak Base Mean A weak base is one which doesn't convert fully into hydroxide ions in solution. Here is the definition of a weak base as the term is. A weak base is one that does not completely dissociate into its ions in water. When a weak base reacts with water, the position of. A weak base is a base that is partially. What Does Weak Base Mean.
From gamesmartz.com
Weak Base Easy to Understand What Does Weak Base Mean A weak base is a base that is partially dissociated in an aqueous solution. A weak base is one which doesn't convert fully into hydroxide ions in solution. When a weak base reacts with water, the position of. Here is the definition of a weak base as the term is. A weak base is one that does not completely dissociate. What Does Weak Base Mean.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6973259 What Does Weak Base Mean A weak base is one that does not completely dissociate into its ions in water. Here is the definition of a weak base as the term is. A weak base is one which doesn't convert fully into hydroxide ions in solution. A weak base is a base that is partially dissociated in an aqueous solution. When a weak base reacts. What Does Weak Base Mean.