Bromine And Chlorine Balanced Equation . (c)€€€€ bromine can be extracted from seawater. The dissolved bromide ions are reacted with chlorine. In this equation, the cl and br have swapped places: Balance a chemical equation when given the unbalanced equation. Bromine + chlorine = bromine monochloride. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. Derive chemical equations from narrative descriptions of chemical reactions. Multiply coefficient for brcl by 2 1 br 2 + 1 cl 2 = 2 brcl cl is balanced: This brown colour is the displaced bromine. 2 atoms in reagents and 2 atoms in. Bromine and chloride ions are formed. In order to balance br on both sides we: Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear.
from www.youtube.com
To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. Chlorine + sodium bromide → sodium. Balance a chemical equation when given the unbalanced equation. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. Explain the role of the law of conservation of mass in a chemical reaction. Bromine + chlorine = bromine monochloride. In order to balance br on both sides we: Bromine and chloride ions are formed. (c)€€€€ bromine can be extracted from seawater.
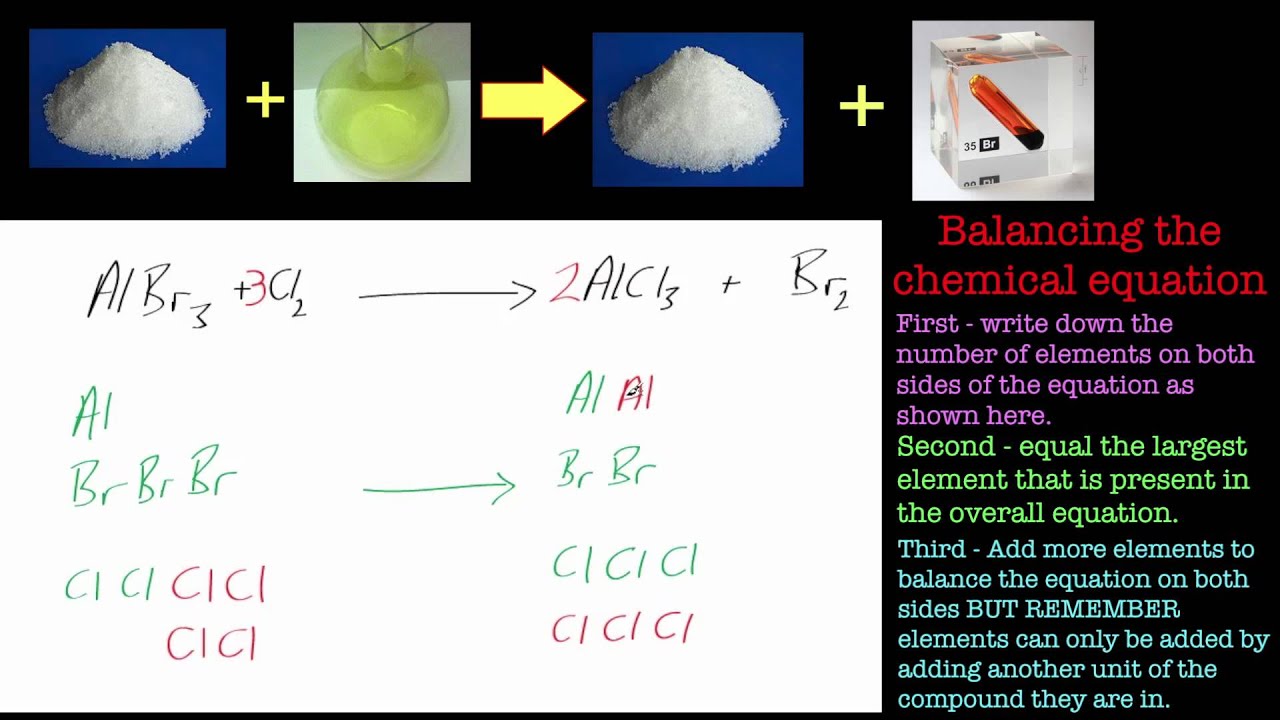
Balancing Chemical Equations. Part 4 Aluminium bromide and chlorine
Bromine And Chlorine Balanced Equation Explain the role of the law of conservation of mass in a chemical reaction. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Derive chemical equations from narrative descriptions of chemical reactions. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. The balanced equation will appear. Chlorine + sodium bromide → sodium. Explain the roles of subscripts and coefficients in chemical equations. Explain the role of the law of conservation of mass in a chemical reaction. 2 atoms in reagents and 2 atoms in. The chlorine has gone to form sodium chloride. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. Multiply coefficient for brcl by 2 1 br 2 + 1 cl 2 = 2 brcl cl is balanced: In this equation, the cl and br have swapped places: Balance a chemical equation when given the unbalanced equation. Bromine + chlorine = bromine monochloride. Write and balance chemical equations in molecular, total.
From www.numerade.com
SOLVEDGaseous chlorine will displace bromide ion from an aqueous Bromine And Chlorine Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Bromine + chlorine = bromine monochloride. Derive chemical equations. Bromine And Chlorine Balanced Equation.
From www.chegg.com
Solved The equilibrium constant for the reaction of bromine Bromine And Chlorine Balanced Equation Chlorine + sodium bromide → sodium. This brown colour is the displaced bromine. (c)€€€€ bromine can be extracted from seawater. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Balance a chemical equation when given the unbalanced equation. Multiply coefficient for brcl by 2 1 br 2 + 1 cl 2 =. Bromine And Chlorine Balanced Equation.
From www.youtube.com
Type of Reaction for NaBr + Cl2 = NaCl + Br2 YouTube Bromine And Chlorine Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. 2 atoms in reagents and 2 atoms in. Bromine and chloride ions are formed. Balance a chemical equation when given the unbalanced equation. In this. Bromine And Chlorine Balanced Equation.
From www.youtube.com
Balancing Chemical Equations. Part 4 Aluminium bromide and chlorine Bromine And Chlorine Balanced Equation Balance a chemical equation when given the unbalanced equation. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. This brown colour is the displaced bromine. Derive chemical equations from narrative descriptions of chemical reactions. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. Bromine +. Bromine And Chlorine Balanced Equation.
From slideplayer.com
Chemical Equations & Reactions ppt download Bromine And Chlorine Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Balance a chemical equation when given the unbalanced equation. Bromine and chloride ions are formed. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Br + cl = brcl is a synthesis reaction where. Bromine And Chlorine Balanced Equation.
From www.numerade.com
SOLVEDBromine is obtained from brine wells. The process involves Bromine And Chlorine Balanced Equation In this equation, the cl and br have swapped places: Chlorine + sodium bromide → sodium. Bromine and chloride ions are formed. 2 atoms in reagents and 2 atoms in. The chlorine has gone to form sodium chloride. Balance a chemical equation when given the unbalanced equation. This brown colour is the displaced bromine. Write a balanced equation for the. Bromine And Chlorine Balanced Equation.
From exoexqcpv.blob.core.windows.net
Bromine Vs Chlorine Selectivity at Denise Perez blog Bromine And Chlorine Balanced Equation In order to balance br on both sides we: Explain the roles of subscripts and coefficients in chemical equations. The balanced equation will appear. Bromine + chlorine = bromine monochloride. This brown colour is the displaced bromine. The dissolved bromide ions are reacted with chlorine. Write and balance chemical equations in molecular, total. Derive chemical equations from narrative descriptions of. Bromine And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Chlorine can replace bromine in bromide compounds, forming Bromine And Chlorine Balanced Equation Chlorine + sodium bromide → sodium. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Multiply coefficient for brcl by 2 1 br 2 + 1 cl 2 = 2 brcl cl is balanced: Write and balance chemical equations in molecular, total. Br + cl = brcl is a synthesis reaction where. Bromine And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Aqueous sodium chloride (NaCl) and liquid bromine (Brz are Bromine And Chlorine Balanced Equation Balance a chemical equation when given the unbalanced equation. In order to balance br on both sides we: In this equation, the cl and br have swapped places: Explain the role of the law of conservation of mass in a chemical reaction. The balanced equation will appear. Explain the roles of subscripts and coefficients in chemical equations. Multiply coefficient for. Bromine And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Sodium chloride reacts with bromine gas, mathrm{Br}_{2}, to Bromine And Chlorine Balanced Equation The chlorine has gone to form sodium chloride. Bromine + chlorine = bromine monochloride. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. This brown colour is the displaced bromine. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. 2 atoms in reagents and. Bromine And Chlorine Balanced Equation.
From socratic.org
What is the balanced chemical equation for this reaction Gaseous Bromine And Chlorine Balanced Equation The balanced equation will appear. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. 2 atoms in reagents and 2 atoms in. Explain the roles of subscripts and coefficients in chemical equations. The chlorine has gone to form sodium chloride. Chlorine + sodium bromide → sodium. In order to balance br. Bromine And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Part A Enter a balanced equation for the reaction of chlorine Bromine And Chlorine Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Explain the role of the law of conservation of mass in a chemical reaction. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. Multiply coefficient for brcl by 2 1 br 2 + 1 cl. Bromine And Chlorine Balanced Equation.
From slideplayer.com
Chemical Reactions Chapter ppt download Bromine And Chlorine Balanced Equation Derive chemical equations from narrative descriptions of chemical reactions. The dissolved bromide ions are reacted with chlorine. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Chlorine + sodium bromide → sodium. In this equation, the cl and br have swapped places: Bromine and chloride ions are formed. (c)€€€€ bromine can be. Bromine And Chlorine Balanced Equation.
From www.slideserve.com
PPT Balancing Equations ANSWER KEY PowerPoint Presentation, free Bromine And Chlorine Balanced Equation Write and balance chemical equations in molecular, total. In order to balance br on both sides we: The dissolved bromide ions are reacted with chlorine. The balanced equation will appear. In this equation, the cl and br have swapped places: (c)€€€€ bromine can be extracted from seawater. Derive chemical equations from narrative descriptions of chemical reactions. Explain the role of. Bromine And Chlorine Balanced Equation.
From slideplayer.com
Chemical Reactions. ppt download Bromine And Chlorine Balanced Equation Write and balance chemical equations in molecular, total. The balanced equation will appear. 2 atoms in reagents and 2 atoms in. The chlorine has gone to form sodium chloride. Chlorine + sodium bromide → sodium. Bromine + chlorine = bromine monochloride. Derive chemical equations from narrative descriptions of chemical reactions. The dissolved bromide ions are reacted with chlorine. In order. Bromine And Chlorine Balanced Equation.
From slideplayer.com
Balancing Equations Mr. Sader. ppt download Bromine And Chlorine Balanced Equation (c)€€€€ bromine can be extracted from seawater. Chlorine + sodium bromide → sodium. The chlorine has gone to form sodium chloride. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. This brown colour is the displaced bromine. The dissolved bromide ions are reacted with chlorine. Br + cl = brcl is. Bromine And Chlorine Balanced Equation.
From www.numerade.com
SOLVED 38. Write balanced chemical equation for the following reaction Bromine And Chlorine Balanced Equation Explain the role of the law of conservation of mass in a chemical reaction. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. In order to balance br on both sides we: Derive chemical equations from narrative descriptions of chemical reactions. Explain the roles of subscripts and coefficients in chemical equations. The chlorine. Bromine And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Potassium bromide + chlorine = potassium chloride + bromine Bromine And Chlorine Balanced Equation (c)€€€€ bromine can be extracted from seawater. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Explain the role of the law of conservation of mass in a chemical reaction. In this equation, the cl and br have swapped places: The balanced equation will appear. Derive chemical equations from narrative descriptions of. Bromine And Chlorine Balanced Equation.
From socratic.org
How do you write the equation for this reaction Aluminum bromide and Bromine And Chlorine Balanced Equation This brown colour is the displaced bromine. Bromine + chlorine = bromine monochloride. 2 atoms in reagents and 2 atoms in. Explain the roles of subscripts and coefficients in chemical equations. The dissolved bromide ions are reacted with chlorine. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Br + cl =. Bromine And Chlorine Balanced Equation.
From www.chegg.com
Solved Part A Write a balanced equation for the reaction of Bromine And Chlorine Balanced Equation The dissolved bromide ions are reacted with chlorine. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The chlorine has gone to form sodium chloride. Bromine + chlorine = bromine monochloride. Explain the role of the law of conservation of mass in a chemical reaction. Bromine and chloride ions are formed. Explain. Bromine And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Liquid bromine and aqueous sodium chloride are produced by the Bromine And Chlorine Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Explain the roles of subscripts and coefficients in chemical equations. In this equation, the cl and br have swapped places: In order to balance br on both sides we: Bromine and chloride ions are formed. Chlorine + sodium bromide → sodium. Multiply coefficient. Bromine And Chlorine Balanced Equation.
From www.youtube.com
How to Balance KBr + Cl2 = KCl + Br2 (Potassium bromide + Chlorine gas Bromine And Chlorine Balanced Equation Write and balance chemical equations in molecular, total. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. The balanced equation will appear. In order to balance br on both sides we: The dissolved bromide ions are reacted with chlorine. Bromine and chloride ions are formed. To balance a chemical equation, enter. Bromine And Chlorine Balanced Equation.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Bromine And Chlorine Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. Multiply coefficient for brcl by 2 1 br 2 + 1 cl 2 = 2 brcl cl is balanced: Bromine + chlorine = bromine monochloride. The balanced equation will appear. Write and balance chemical equations in molecular, total. Explain the role of the law of conservation of mass in a. Bromine And Chlorine Balanced Equation.
From www.numerade.com
SOLVEDWrite balanced equations for the reaction of chlorine with the Bromine And Chlorine Balanced Equation The chlorine has gone to form sodium chloride. The dissolved bromide ions are reacted with chlorine. Explain the roles of subscripts and coefficients in chemical equations. Chlorine + sodium bromide → sodium. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Explain the role of the law of conservation of mass in. Bromine And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce Bromine And Chlorine Balanced Equation (c)€€€€ bromine can be extracted from seawater. 2 atoms in reagents and 2 atoms in. Multiply coefficient for brcl by 2 1 br 2 + 1 cl 2 = 2 brcl cl is balanced: This brown colour is the displaced bromine. The chlorine has gone to form sodium chloride. Bromine + chlorine = bromine monochloride. To balance a chemical equation,. Bromine And Chlorine Balanced Equation.
From www.youtube.com
Chlorine And Sodium Bromide Make Sodium Chloride And Bromine YouTube Bromine And Chlorine Balanced Equation In this equation, the cl and br have swapped places: Chlorine + sodium bromide → sodium. This brown colour is the displaced bromine. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Multiply coefficient for brcl by 2 1 br 2 + 1 cl 2 = 2 brcl cl is balanced: Br. Bromine And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for Combustion of octane Combustion Bromine And Chlorine Balanced Equation In this equation, the cl and br have swapped places: The chlorine has gone to form sodium chloride. The balanced equation will appear. The dissolved bromide ions are reacted with chlorine. (c)€€€€ bromine can be extracted from seawater. Multiply coefficient for brcl by 2 1 br 2 + 1 cl 2 = 2 brcl cl is balanced: Explain the roles. Bromine And Chlorine Balanced Equation.
From slideplayer.com
Biology Dr. Altstiel Naples American High School ppt download Bromine And Chlorine Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Bromine and chloride ions are formed. Write and balance chemical equations in molecular, total. This brown colour is the displaced bromine. Bromine + chlorine = bromine monochloride. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and.. Bromine And Chlorine Balanced Equation.
From slideplayer.com
Balancing chemical equations ppt download Bromine And Chlorine Balanced Equation Bromine and chloride ions are formed. In order to balance br on both sides we: The chlorine has gone to form sodium chloride. (c)€€€€ bromine can be extracted from seawater. This brown colour is the displaced bromine. 2 atoms in reagents and 2 atoms in. Explain the role of the law of conservation of mass in a chemical reaction. The. Bromine And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Determine the Electron Configurations for the following Bromine And Chlorine Balanced Equation Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. (c)€€€€ bromine can be extracted from seawater. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. This brown. Bromine And Chlorine Balanced Equation.
From slideplayer.com
Chemical Equations Chapter ppt download Bromine And Chlorine Balanced Equation The chlorine has gone to form sodium chloride. Derive chemical equations from narrative descriptions of chemical reactions. Write and balance chemical equations in molecular, total. Multiply coefficient for brcl by 2 1 br 2 + 1 cl 2 = 2 brcl cl is balanced: Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen,. Bromine And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Sodium chloride reacts with bromine gas, Br2, to produce sodium Bromine And Chlorine Balanced Equation Write and balance chemical equations in molecular, total. In this equation, the cl and br have swapped places: Bromine + chlorine = bromine monochloride. Derive chemical equations from narrative descriptions of chemical reactions. Explain the role of the law of conservation of mass in a chemical reaction. The chlorine has gone to form sodium chloride. To balance a chemical equation,. Bromine And Chlorine Balanced Equation.
From brainly.com
Balance the chemical equation. Based on the equation, how many grams of Bromine And Chlorine Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Multiply coefficient for brcl by 2 1 br 2 + 1 cl 2 = 2 brcl cl is balanced: Derive chemical equations from narrative descriptions of chemical reactions. The chlorine has gone to form sodium chloride. In this equation, the cl and. Bromine And Chlorine Balanced Equation.
From slideplayer.com
Biology Dr. Altstiel Naples American High School ppt download Bromine And Chlorine Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. (c)€€€€ bromine can be extracted from seawater. Explain the roles of subscripts and coefficients in chemical equations. 2 atoms in reagents and 2 atoms in. Balance a chemical equation when given the unbalanced equation. Chlorine + sodium bromide. Bromine And Chlorine Balanced Equation.
From www.numerade.com
SOLVED Consider the reaction of bromide and chlorine Balance the Bromine And Chlorine Balanced Equation The dissolved bromide ions are reacted with chlorine. Bromine and chloride ions are formed. Multiply coefficient for brcl by 2 1 br 2 + 1 cl 2 = 2 brcl cl is balanced: (c)€€€€ bromine can be extracted from seawater. Balance a chemical equation when given the unbalanced equation. Bromine + chlorine = bromine monochloride. Derive chemical equations from narrative. Bromine And Chlorine Balanced Equation.