Bromine Plus Fluorine . One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to form a haloalkane. Fluorine we’ll leave until the end. Bromine fluoride may refer to several compounds with the elements bromine and fluorine: Let’s look at bromine and iodine. What about fluorine (f2), bromine (br2), and iodine (i2)? It reacts with many metals and metal oxides to form similar ionized entities; Chlorine and bromine are strong enough oxidising agents to oxidise iron(ii) ions. It is used in organic chemistry as a fluorinating agent. This reaction is very important in. With some others it forms the metal fluoride plus free bromine and oxygen. Wherever you have solutions, fluorine will react with the water. Chlorine isn’t the only halogen on the periodic table.
from www.numerade.com
With some others it forms the metal fluoride plus free bromine and oxygen. Let’s look at bromine and iodine. This reaction is very important in. Chlorine isn’t the only halogen on the periodic table. What about fluorine (f2), bromine (br2), and iodine (i2)? It is used in organic chemistry as a fluorinating agent. Wherever you have solutions, fluorine will react with the water. One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to form a haloalkane. Bromine fluoride may refer to several compounds with the elements bromine and fluorine: Chlorine and bromine are strong enough oxidising agents to oxidise iron(ii) ions.
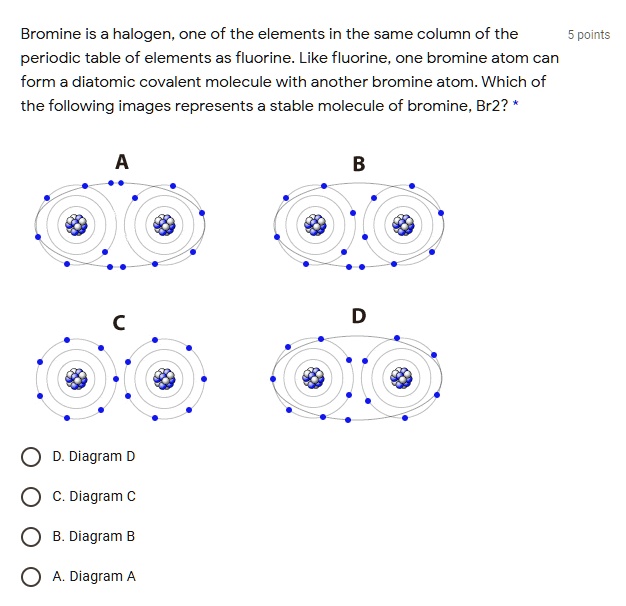
Bromine is a halogen; one of the elements in the same column of the
Bromine Plus Fluorine What about fluorine (f2), bromine (br2), and iodine (i2)? What about fluorine (f2), bromine (br2), and iodine (i2)? Let’s look at bromine and iodine. It reacts with many metals and metal oxides to form similar ionized entities; Fluorine we’ll leave until the end. Bromine fluoride may refer to several compounds with the elements bromine and fluorine: It is used in organic chemistry as a fluorinating agent. Chlorine isn’t the only halogen on the periodic table. One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to form a haloalkane. Wherever you have solutions, fluorine will react with the water. Chlorine and bromine are strong enough oxidising agents to oxidise iron(ii) ions. This reaction is very important in. With some others it forms the metal fluoride plus free bromine and oxygen.
From www.whitecorals.com
Sangokai ChemIndividual BrF Bromine/Fluorine solution 1000ml buy online Bromine Plus Fluorine What about fluorine (f2), bromine (br2), and iodine (i2)? It reacts with many metals and metal oxides to form similar ionized entities; Let’s look at bromine and iodine. Chlorine isn’t the only halogen on the periodic table. Fluorine we’ll leave until the end. One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for. Bromine Plus Fluorine.
From animalia-life.club
Fluorine Atom 3d Model Bromine Plus Fluorine Let’s look at bromine and iodine. With some others it forms the metal fluoride plus free bromine and oxygen. It reacts with many metals and metal oxides to form similar ionized entities; Wherever you have solutions, fluorine will react with the water. One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a. Bromine Plus Fluorine.
From joelgordon.photoshelter.com
Chlorine Bromine Iodine Joel Gordon Photography Bromine Plus Fluorine Wherever you have solutions, fluorine will react with the water. Bromine fluoride may refer to several compounds with the elements bromine and fluorine: Fluorine we’ll leave until the end. With some others it forms the metal fluoride plus free bromine and oxygen. What about fluorine (f2), bromine (br2), and iodine (i2)? It reacts with many metals and metal oxides to. Bromine Plus Fluorine.
From www.alamy.com
Fluorine, Bromine and Chlorine Molecular Model of Atom. Vector Bromine Plus Fluorine It is used in organic chemistry as a fluorinating agent. What about fluorine (f2), bromine (br2), and iodine (i2)? One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to form a haloalkane. Bromine fluoride may refer to several compounds with the elements bromine and. Bromine Plus Fluorine.
From socratic.org
Why does fluorine have a higher ionization energy than bromine? Socratic Bromine Plus Fluorine This reaction is very important in. Bromine fluoride may refer to several compounds with the elements bromine and fluorine: With some others it forms the metal fluoride plus free bromine and oxygen. One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to form a. Bromine Plus Fluorine.
From joieqcwyg.blob.core.windows.net
Bromine Plus Ion at David Martinelli blog Bromine Plus Fluorine Chlorine isn’t the only halogen on the periodic table. Wherever you have solutions, fluorine will react with the water. Let’s look at bromine and iodine. With some others it forms the metal fluoride plus free bromine and oxygen. It is used in organic chemistry as a fluorinating agent. What about fluorine (f2), bromine (br2), and iodine (i2)? It reacts with. Bromine Plus Fluorine.
From en.futongchem.cn
Shandong Futong Chemical Co., Ltd._pfluorotoluene,Bromine Bromine Plus Fluorine Bromine fluoride may refer to several compounds with the elements bromine and fluorine: One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to form a haloalkane. This reaction is very important in. Wherever you have solutions, fluorine will react with the water. Chlorine isn’t. Bromine Plus Fluorine.
From imgbin.com
Concise Encyclopedia Chemistry Iodine Monofluoride Chlorine Bromine Plus Fluorine It reacts with many metals and metal oxides to form similar ionized entities; What about fluorine (f2), bromine (br2), and iodine (i2)? One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to form a haloalkane. Chlorine and bromine are strong enough oxidising agents to. Bromine Plus Fluorine.
From www.alamy.com
Molecular Model of Fluorine (F2) Molecule. Vector Illustration Stock Bromine Plus Fluorine This reaction is very important in. Wherever you have solutions, fluorine will react with the water. It reacts with many metals and metal oxides to form similar ionized entities; What about fluorine (f2), bromine (br2), and iodine (i2)? Chlorine isn’t the only halogen on the periodic table. Bromine fluoride may refer to several compounds with the elements bromine and fluorine:. Bromine Plus Fluorine.
From www.researchgate.net
Recoveries of bromine (), chlorine (), fluorine (), iodine (), and Bromine Plus Fluorine With some others it forms the metal fluoride plus free bromine and oxygen. One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to form a haloalkane. What about fluorine (f2), bromine (br2), and iodine (i2)? Fluorine we’ll leave until the end. Bromine fluoride may. Bromine Plus Fluorine.
From www.pinterest.com
Fluorine Halogens Chemistry, Element chemistry, Electron configuration Bromine Plus Fluorine Fluorine we’ll leave until the end. What about fluorine (f2), bromine (br2), and iodine (i2)? Chlorine isn’t the only halogen on the periodic table. Bromine fluoride may refer to several compounds with the elements bromine and fluorine: Chlorine and bromine are strong enough oxidising agents to oxidise iron(ii) ions. Let’s look at bromine and iodine. It reacts with many metals. Bromine Plus Fluorine.
From ar.inspiredpencil.com
Fluorine Molecular Structure Bromine Plus Fluorine Let’s look at bromine and iodine. Bromine fluoride may refer to several compounds with the elements bromine and fluorine: What about fluorine (f2), bromine (br2), and iodine (i2)? It is used in organic chemistry as a fluorinating agent. With some others it forms the metal fluoride plus free bromine and oxygen. Chlorine and bromine are strong enough oxidising agents to. Bromine Plus Fluorine.
From dxodugneq.blob.core.windows.net
Bromine Uses In Medicine at Carl Robinson blog Bromine Plus Fluorine Bromine fluoride may refer to several compounds with the elements bromine and fluorine: Fluorine we’ll leave until the end. Chlorine isn’t the only halogen on the periodic table. One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to form a haloalkane. Chlorine and bromine. Bromine Plus Fluorine.
From www.dreamstime.com
Bromine Fluoride Molecule Stock Image Image 8439131 Bromine Plus Fluorine This reaction is very important in. Chlorine and bromine are strong enough oxidising agents to oxidise iron(ii) ions. It reacts with many metals and metal oxides to form similar ionized entities; Chlorine isn’t the only halogen on the periodic table. Let’s look at bromine and iodine. What about fluorine (f2), bromine (br2), and iodine (i2)? One of these reactions is. Bromine Plus Fluorine.
From mungfali.com
Bromine Pentafluoride Lewis Structure Bromine Plus Fluorine Let’s look at bromine and iodine. This reaction is very important in. Wherever you have solutions, fluorine will react with the water. One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to form a haloalkane. What about fluorine (f2), bromine (br2), and iodine (i2)?. Bromine Plus Fluorine.
From en.deltachem.net
The bromine fluorine benzene Bromine Plus Fluorine Chlorine and bromine are strong enough oxidising agents to oxidise iron(ii) ions. With some others it forms the metal fluoride plus free bromine and oxygen. Chlorine isn’t the only halogen on the periodic table. It reacts with many metals and metal oxides to form similar ionized entities; Bromine fluoride may refer to several compounds with the elements bromine and fluorine:. Bromine Plus Fluorine.
From joieqcwyg.blob.core.windows.net
Bromine Plus Ion at David Martinelli blog Bromine Plus Fluorine Wherever you have solutions, fluorine will react with the water. This reaction is very important in. One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to form a haloalkane. It is used in organic chemistry as a fluorinating agent. It reacts with many metals. Bromine Plus Fluorine.
From www.numerade.com
SOLVED 'Consider the cyclohexane below H H Br In this conformation Bromine Plus Fluorine One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to form a haloalkane. Let’s look at bromine and iodine. Chlorine isn’t the only halogen on the periodic table. It reacts with many metals and metal oxides to form similar ionized entities; Fluorine we’ll leave. Bromine Plus Fluorine.
From stock.adobe.com
Vecteur Stock Diatomic molecules diagram shows elements that exist as Bromine Plus Fluorine This reaction is very important in. Bromine fluoride may refer to several compounds with the elements bromine and fluorine: What about fluorine (f2), bromine (br2), and iodine (i2)? Chlorine isn’t the only halogen on the periodic table. It is used in organic chemistry as a fluorinating agent. Chlorine and bromine are strong enough oxidising agents to oxidise iron(ii) ions. One. Bromine Plus Fluorine.
From www.masterorganicchemistry.com
Introduction to Free Radical Substitution Reactions Master Organic Bromine Plus Fluorine Fluorine we’ll leave until the end. Let’s look at bromine and iodine. Chlorine isn’t the only halogen on the periodic table. With some others it forms the metal fluoride plus free bromine and oxygen. It is used in organic chemistry as a fluorinating agent. This reaction is very important in. One of these reactions is halogenation, or the substitution of. Bromine Plus Fluorine.
From joieqcwyg.blob.core.windows.net
Bromine Plus Ion at David Martinelli blog Bromine Plus Fluorine Bromine fluoride may refer to several compounds with the elements bromine and fluorine: With some others it forms the metal fluoride plus free bromine and oxygen. It is used in organic chemistry as a fluorinating agent. What about fluorine (f2), bromine (br2), and iodine (i2)? Chlorine isn’t the only halogen on the periodic table. Let’s look at bromine and iodine.. Bromine Plus Fluorine.
From www.alamy.com
Molecular Model of Fluorine (F2) Molecule. Vector Illustration Stock Bromine Plus Fluorine It is used in organic chemistry as a fluorinating agent. Chlorine and bromine are strong enough oxidising agents to oxidise iron(ii) ions. Bromine fluoride may refer to several compounds with the elements bromine and fluorine: Fluorine we’ll leave until the end. Wherever you have solutions, fluorine will react with the water. What about fluorine (f2), bromine (br2), and iodine (i2)?. Bromine Plus Fluorine.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Bromine Plus Fluorine It reacts with many metals and metal oxides to form similar ionized entities; Chlorine and bromine are strong enough oxidising agents to oxidise iron(ii) ions. Chlorine isn’t the only halogen on the periodic table. It is used in organic chemistry as a fluorinating agent. Wherever you have solutions, fluorine will react with the water. Bromine fluoride may refer to several. Bromine Plus Fluorine.
From peguys.com
Fluorine Chlorine Bromine Iodine Low Pressure Ampoules set in Periodic Bromine Plus Fluorine Chlorine isn’t the only halogen on the periodic table. Fluorine we’ll leave until the end. Let’s look at bromine and iodine. Bromine fluoride may refer to several compounds with the elements bromine and fluorine: Wherever you have solutions, fluorine will react with the water. It is used in organic chemistry as a fluorinating agent. What about fluorine (f2), bromine (br2),. Bromine Plus Fluorine.
From www.walmart.com
In The Swim 1 Inch Bromine Plus Tablet Sanitizer for Spas, Hot Tubs, or Bromine Plus Fluorine Wherever you have solutions, fluorine will react with the water. Bromine fluoride may refer to several compounds with the elements bromine and fluorine: What about fluorine (f2), bromine (br2), and iodine (i2)? One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to form a. Bromine Plus Fluorine.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Plus Fluorine Chlorine and bromine are strong enough oxidising agents to oxidise iron(ii) ions. Fluorine we’ll leave until the end. It is used in organic chemistry as a fluorinating agent. One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to form a haloalkane. Wherever you have. Bromine Plus Fluorine.
From slideplayer.com
Chemical Families. ppt download Bromine Plus Fluorine With some others it forms the metal fluoride plus free bromine and oxygen. Wherever you have solutions, fluorine will react with the water. Chlorine and bromine are strong enough oxidising agents to oxidise iron(ii) ions. One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2). Bromine Plus Fluorine.
From www.numerade.com
Bromine is a halogen; one of the elements in the same column of the Bromine Plus Fluorine Wherever you have solutions, fluorine will react with the water. Chlorine isn’t the only halogen on the periodic table. Let’s look at bromine and iodine. Fluorine we’ll leave until the end. Chlorine and bromine are strong enough oxidising agents to oxidise iron(ii) ions. One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for. Bromine Plus Fluorine.
From animalia-life.club
Fluorine Atom 3d Model Bromine Plus Fluorine Let’s look at bromine and iodine. Fluorine we’ll leave until the end. With some others it forms the metal fluoride plus free bromine and oxygen. What about fluorine (f2), bromine (br2), and iodine (i2)? Bromine fluoride may refer to several compounds with the elements bromine and fluorine: Wherever you have solutions, fluorine will react with the water. Chlorine isn’t the. Bromine Plus Fluorine.
From www.alamy.com
Fluorine Periodic Table of the Elements Vector illustration eps 10 Bromine Plus Fluorine It reacts with many metals and metal oxides to form similar ionized entities; It is used in organic chemistry as a fluorinating agent. Bromine fluoride may refer to several compounds with the elements bromine and fluorine: What about fluorine (f2), bromine (br2), and iodine (i2)? Chlorine isn’t the only halogen on the periodic table. This reaction is very important in.. Bromine Plus Fluorine.
From nap.nationalacademies.org
Table of Isotopes of Fluorine, Chlorine, Bromine and Iodine The Bromine Plus Fluorine This reaction is very important in. Let’s look at bromine and iodine. With some others it forms the metal fluoride plus free bromine and oxygen. What about fluorine (f2), bromine (br2), and iodine (i2)? Bromine fluoride may refer to several compounds with the elements bromine and fluorine: It reacts with many metals and metal oxides to form similar ionized entities;. Bromine Plus Fluorine.
From nap.nationalacademies.org
Table of Isotopes of Fluorine, Chlorine, Bromine and Iodine The Bromine Plus Fluorine This reaction is very important in. Wherever you have solutions, fluorine will react with the water. Chlorine and bromine are strong enough oxidising agents to oxidise iron(ii) ions. Chlorine isn’t the only halogen on the periodic table. Bromine fluoride may refer to several compounds with the elements bromine and fluorine: What about fluorine (f2), bromine (br2), and iodine (i2)? One. Bromine Plus Fluorine.
From www.alamy.com
Chlorine atomic structure Cut Out Stock Images & Pictures Alamy Bromine Plus Fluorine Bromine fluoride may refer to several compounds with the elements bromine and fluorine: It reacts with many metals and metal oxides to form similar ionized entities; What about fluorine (f2), bromine (br2), and iodine (i2)? Let’s look at bromine and iodine. Fluorine we’ll leave until the end. One of these reactions is halogenation, or the substitution of a single hydrogen. Bromine Plus Fluorine.
From www.anyrgb.com
Pblock, homonuclear Molecule, bromine Dioxide, iodine127, diatomic Bromine Plus Fluorine Let’s look at bromine and iodine. What about fluorine (f2), bromine (br2), and iodine (i2)? This reaction is very important in. Bromine fluoride may refer to several compounds with the elements bromine and fluorine: Chlorine isn’t the only halogen on the periodic table. Fluorine we’ll leave until the end. With some others it forms the metal fluoride plus free bromine. Bromine Plus Fluorine.
From www.alamy.com
halogens in gas jars Fl Cl Br I fluorine chlorine bromine iodine Stock Bromine Plus Fluorine It is used in organic chemistry as a fluorinating agent. One of these reactions is halogenation, or the substitution of a single hydrogen on the alkane for a single halogen (cl 2 or br 2) to form a haloalkane. It reacts with many metals and metal oxides to form similar ionized entities; Bromine fluoride may refer to several compounds with. Bromine Plus Fluorine.