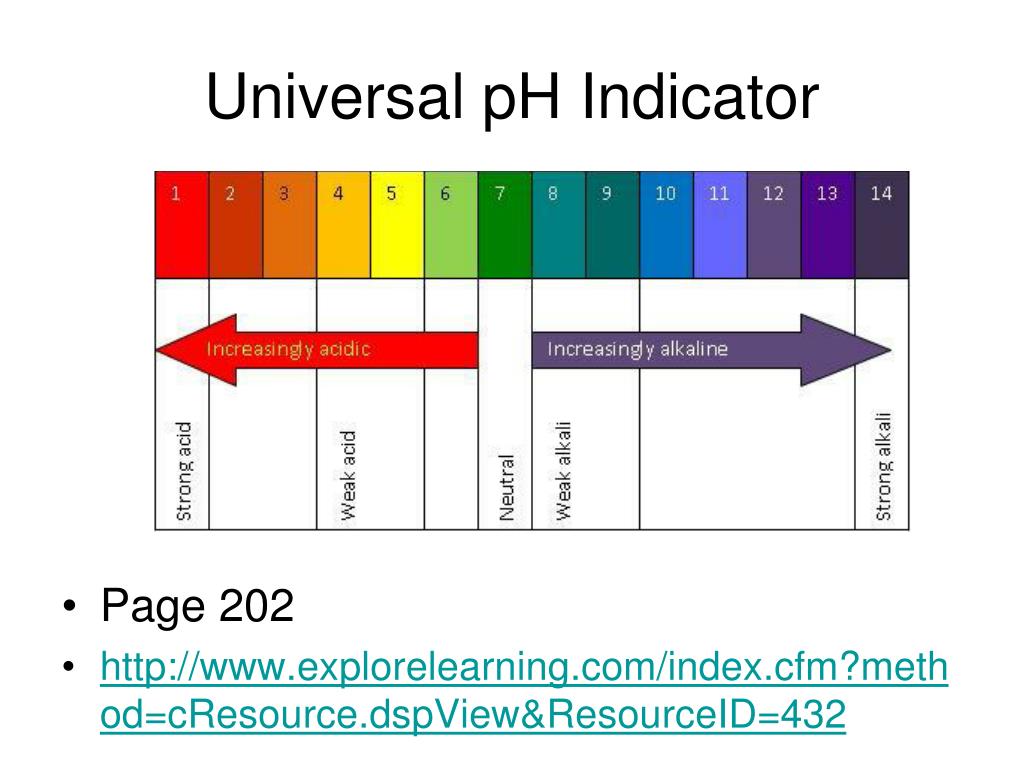
Indicators For Strong Base . In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. This page assumes that you know about ph curves for all the. A ph curve is found if the ph of the solution being. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. A method, such as an indicator, must be used in a titration to locate the equivalence point. When titrating, acid can either be added to base or base can be added to acid, both will result. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments.
from www.slideserve.com
An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. When titrating, acid can either be added to base or base can be added to acid, both will result. This page assumes that you know about ph curves for all the. A ph curve is found if the ph of the solution being. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. A method, such as an indicator, must be used in a titration to locate the equivalence point.
PPT Chapter 5 Acids and Bases PowerPoint Presentation, free download
Indicators For Strong Base An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. A method, such as an indicator, must be used in a titration to locate the equivalence point. When titrating, acid can either be added to base or base can be added to acid, both will result. This page assumes that you know about ph curves for all the. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. A ph curve is found if the ph of the solution being. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid.
From www.teachoo.com
Strength of Acids and Bases (How to find it?) Chemistry Teachoo Indicators For Strong Base When titrating, acid can either be added to base or base can be added to acid, both will result. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. A method, such as an indicator, must be used in a titration to locate the equivalence point. An aqueous. Indicators For Strong Base.
From saylordotorg.github.io
AcidBase Titrations Indicators For Strong Base When titrating, acid can either be added to base or base can be added to acid, both will result. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. This page assumes that you know about ph curves for all the. A method, such as an indicator, must. Indicators For Strong Base.
From www.chegg.com
Solved 1. Figure 1 shows the titration curves of four Indicators For Strong Base When titrating, acid can either be added to base or base can be added to acid, both will result. This page assumes that you know about ph curves for all the. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. An aqueous solution of hydrochloric acid, hcl. Indicators For Strong Base.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Indicators For Strong Base When titrating, acid can either be added to base or base can be added to acid, both will result. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. A ph curve is found if the ph of the solution being. An aqueous solution. Indicators For Strong Base.
From www.slideshare.net
Acid base titration Indicators For Strong Base An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. This page assumes that you know about ph curves for all the. A method, such as an indicator, must be used in. Indicators For Strong Base.
From study.com
Strong Base Overview, Examples & Characteristics Video & Lesson Indicators For Strong Base A method, such as an indicator, must be used in a titration to locate the equivalence point. A ph curve is found if the ph of the solution being. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. When titrating, acid can either. Indicators For Strong Base.
From chem.libretexts.org
17.3 AcidBase Indicators Chemistry LibreTexts Indicators For Strong Base In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. A method, such as an indicator, must be used in a titration to locate the equivalence point. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. When titrating, acid can either. Indicators For Strong Base.
From chem.libretexts.org
17.3 AcidBase Indicators Chemistry LibreTexts Indicators For Strong Base A method, such as an indicator, must be used in a titration to locate the equivalence point. When titrating, acid can either be added to base or base can be added to acid, both will result. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. This page. Indicators For Strong Base.
From www.slideserve.com
PPT The pH scale PowerPoint Presentation, free download ID4827855 Indicators For Strong Base When titrating, acid can either be added to base or base can be added to acid, both will result. A ph curve is found if the ph of the solution being. This page assumes that you know about ph curves for all the. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a. Indicators For Strong Base.
From slidetodoc.com
ACID BASE TITRATION INDICATORS Titration a method of Indicators For Strong Base This page assumes that you know about ph curves for all the. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. A ph curve is found if the ph of the. Indicators For Strong Base.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Indicators For Strong Base A method, such as an indicator, must be used in a titration to locate the equivalence point. This page assumes that you know about ph curves for all the. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. A ph curve is found if the ph of the solution being. When titrating, acid can either be added. Indicators For Strong Base.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicators For Strong Base An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do.. Indicators For Strong Base.
From byjus.com
Study The pH Change In The Titration Of A Strong Base Using Universal Indicators For Strong Base Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. When titrating, acid can either be added to base or base can be added to acid, both will result. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. In general, for titrations of strong acids. Indicators For Strong Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicators For Strong Base An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. When titrating, acid can either be added to base or base can be added to acid, both will result. A ph curve is found if the ph of the solution being. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures. Indicators For Strong Base.
From joiwaivrn.blob.core.windows.net
Different Indicators For Acids And Bases at Emily Marshall blog Indicators For Strong Base Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid.. Indicators For Strong Base.
From davidswart.wordpress.com
Acids and Bases The Art of Science Indicators For Strong Base This page assumes that you know about ph curves for all the. When titrating, acid can either be added to base or base can be added to acid, both will result. A ph curve is found if the ph of the solution being. A method, such as an indicator, must be used in a titration to locate the equivalence point.. Indicators For Strong Base.
From study.com
Strong Base Definition & Examples Video & Lesson Transcript Indicators For Strong Base An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. A ph curve is found if the ph of the solution being. A method, such as an indicator, must be used in a titration to locate the equivalence point. When titrating, acid can either be added to base or base can be added to acid, both will result.. Indicators For Strong Base.
From guitarscalechart.z28.web.core.windows.net
acids and bases ph scale chart 3.12 acids and bases human biology Indicators For Strong Base In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. A method, such as an indicator, must be used in a titration to locate the equivalence point. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. A ph curve is found. Indicators For Strong Base.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry Indicators For Strong Base When titrating, acid can either be added to base or base can be added to acid, both will result. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. A method, such as an indicator, must. Indicators For Strong Base.
From saylordotorg.github.io
AcidBase Titrations Indicators For Strong Base In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. This page assumes that you know about ph curves for all the. A ph curve is found if the ph of the solution being. Ph indicators are weak acids or weak bases that have. Indicators For Strong Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicators For Strong Base When titrating, acid can either be added to base or base can be added to acid, both will result. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. This page assumes that you know about ph curves for all the. An aqueous solution. Indicators For Strong Base.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicators For Strong Base An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. This page assumes that you know about ph curves for all the. Ph indicators are weak acids or weak bases that have. Indicators For Strong Base.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicators For Strong Base Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. This page assumes that you know about ph curves for all the. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. A method, such as an indicator, must be used in a titration to locate. Indicators For Strong Base.
From www.youtube.com
Strong acidweak base titration indicators YouTube Indicators For Strong Base Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. A method, such as an indicator, must be used in a titration to locate the equivalence point. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about. Indicators For Strong Base.
From www.slideserve.com
PPT Strong AcidWeak Base and Weak Acid Strong Base PowerPoint Indicators For Strong Base A ph curve is found if the ph of the solution being. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments.. Indicators For Strong Base.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicators For Strong Base When titrating, acid can either be added to base or base can be added to acid, both will result. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. A ph curve is found if the ph of the solution being. This page assumes that you know about. Indicators For Strong Base.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Indicators For Strong Base This page assumes that you know about ph curves for all the. When titrating, acid can either be added to base or base can be added to acid, both will result. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. A ph curve. Indicators For Strong Base.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID Indicators For Strong Base A ph curve is found if the ph of the solution being. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. In general, for titrations of strong acids with strong bases (and vice versa), any. Indicators For Strong Base.
From www.slideserve.com
PPT Chapter 5 Acids and Bases PowerPoint Presentation, free download Indicators For Strong Base This page assumes that you know about ph curves for all the. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in between about 4.0 and 10.0 will do. When titrating, acid can either be added to base or. Indicators For Strong Base.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicators For Strong Base This page assumes that you know about ph curves for all the. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. When titrating, acid can either be added to base or base can be added to acid, both will result. A method, such as an indicator, must be used in a titration to locate the equivalence point.. Indicators For Strong Base.
From joiwaivrn.blob.core.windows.net
Different Indicators For Acids And Bases at Emily Marshall blog Indicators For Strong Base When titrating, acid can either be added to base or base can be added to acid, both will result. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a pk in. Indicators For Strong Base.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Indicators For Strong Base A ph curve is found if the ph of the solution being. When titrating, acid can either be added to base or base can be added to acid, both will result. This page assumes that you know about ph curves for all the. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a. Indicators For Strong Base.
From www.walmart.com
Dial Indicator Base Strong Adjustable Universal Cast Iron Indicators For Strong Base A ph curve is found if the ph of the solution being. When titrating, acid can either be added to base or base can be added to acid, both will result. A method, such as an indicator, must be used in a titration to locate the equivalence point. Ph indicators are weak acids or weak bases that have different colors. Indicators For Strong Base.
From www.youtube.com
acids,bases and salts indicators class10 by poonam malhotra YouTube Indicators For Strong Base An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. A ph curve is found if the ph of the solution being. When titrating, acid can either be added to base or base can be added to acid, both will result. In general, for titrations of strong acids with strong bases (and vice versa), any indicator with a. Indicators For Strong Base.
From www.slideserve.com
PPT Acid/Base Indicators PowerPoint Presentation, free download ID Indicators For Strong Base A ph curve is found if the ph of the solution being. An aqueous solution of hydrochloric acid, hcl (aq), is a strong acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. A method, such as an indicator, must be used in a titration to locate. Indicators For Strong Base.