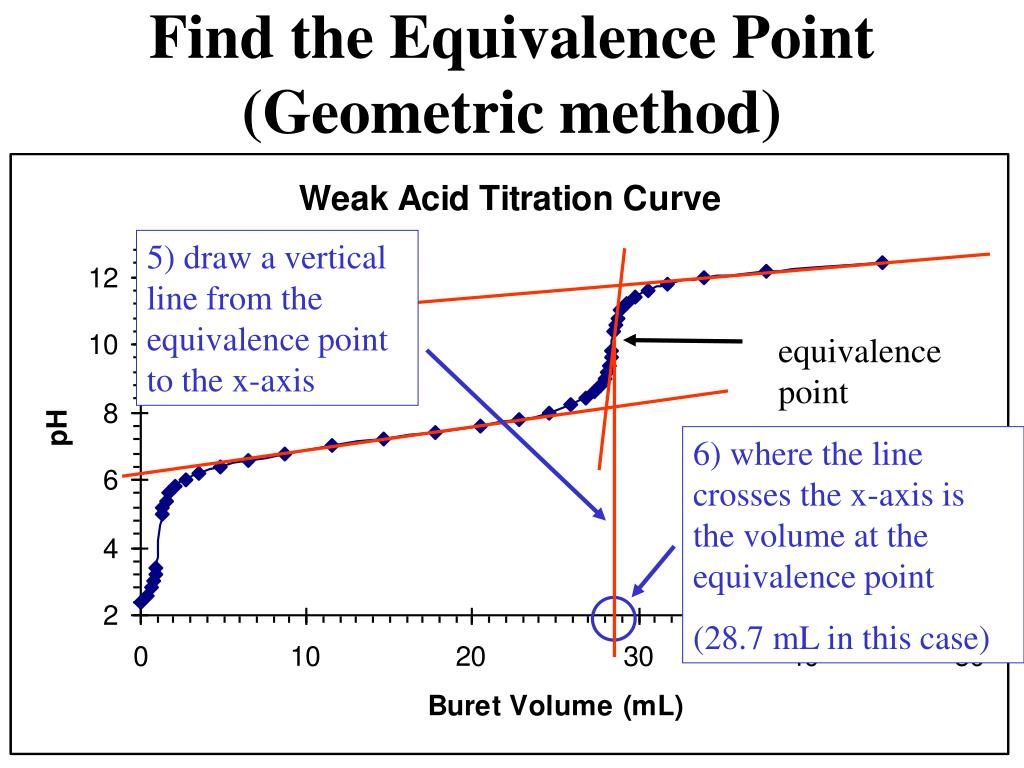
Determine Equivalence Point From Titration Curve . The equivalence point of a titration. \ce{naoh}\) is required to reach the end point when titrated. Postequivalence point (v > 25 ml): Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Equivalence point (v = 25 ml): At the equivalence point, the number. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Sorting out some confusing terms. Suppose that a titration is performed and \(20.70 \: A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. Ph is determined by the amount of excess strong base titrant added; Since both samples are titrated.
from www.slideserve.com
The equivalence point of a titration. Since both samples are titrated. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. \ce{naoh}\) is required to reach the end point when titrated. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. Sorting out some confusing terms. Postequivalence point (v > 25 ml): At the equivalence point, the number. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Equivalence point (v = 25 ml):
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155
Determine Equivalence Point From Titration Curve Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Sorting out some confusing terms. The equivalence point of a titration. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. At the equivalence point, the number. Equivalence point (v = 25 ml): Ph is determined by the amount of excess strong base titrant added; Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Suppose that a titration is performed and \(20.70 \: Since both samples are titrated. Postequivalence point (v > 25 ml): \ce{naoh}\) is required to reach the end point when titrated.
From mavink.com
Acid Base Titration Curve Determine Equivalence Point From Titration Curve A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. Sorting out some confusing terms. Since both samples are titrated. The equivalence point of a titration. Equivalence point (v = 25 ml): \ce{naoh}\) is required to reach the end point when titrated. Equivalence. Determine Equivalence Point From Titration Curve.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Determine Equivalence Point From Titration Curve \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \: Postequivalence point (v > 25 ml): Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Since both samples are titrated. A drastic rise in ph is observed as the solution composition transitions. Determine Equivalence Point From Titration Curve.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Determine Equivalence Point From Titration Curve Since both samples are titrated. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Suppose that a titration is performed and \(20.70 \: Postequivalence point (v > 25 ml): Equivalence point (v = 25 ml): Equivalence point means the point during titration at which the titrant added. Determine Equivalence Point From Titration Curve.
From mungfali.com
Half Equivalence Point On Titration Curve Determine Equivalence Point From Titration Curve At the equivalence point, the number. Sorting out some confusing terms. Ph is determined by the amount of excess strong base titrant added; Postequivalence point (v > 25 ml): The equivalence point of a titration. To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. \ce{naoh}\) is required. Determine Equivalence Point From Titration Curve.
From www.writework.com
Titration of amino acids WriteWork Determine Equivalence Point From Titration Curve To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. At the equivalence point, the number. \ce{naoh}\) is required to reach. Determine Equivalence Point From Titration Curve.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Determine Equivalence Point From Titration Curve Postequivalence point (v > 25 ml): Sorting out some confusing terms. \ce{naoh}\) is required to reach the end point when titrated. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Equivalence point (v = 25 ml): Since both samples are titrated. A drastic rise in ph is observed as the solution. Determine Equivalence Point From Titration Curve.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Determine Equivalence Point From Titration Curve At the equivalence point, the number. Equivalence point (v = 25 ml): Ph is determined by the amount of excess strong base titrant added; The equivalence point of a titration. Suppose that a titration is performed and \(20.70 \: Postequivalence point (v > 25 ml): A drastic rise in ph is observed as the solution composition transitions from acidic to. Determine Equivalence Point From Titration Curve.
From slidetodoc.com
How to Interpret Titration Curves find the equivalence Determine Equivalence Point From Titration Curve \ce{naoh}\) is required to reach the end point when titrated. Ph is determined by the amount of excess strong base titrant added; To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Equivalence point (v = 25 ml): Suppose that a titration is performed and \(20.70 \: Equivalence. Determine Equivalence Point From Titration Curve.
From srkzhxhdjshcc.blogspot.com
How To Find Half Equivalence Point On Titration Curve Excel Is there any good way to find a Determine Equivalence Point From Titration Curve Sorting out some confusing terms. Suppose that a titration is performed and \(20.70 \: A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. To. Determine Equivalence Point From Titration Curve.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free download ID1270033 Determine Equivalence Point From Titration Curve To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Since both samples are titrated. \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \: Postequivalence point (v > 25 ml): Ph is determined by the amount of excess. Determine Equivalence Point From Titration Curve.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Determine Equivalence Point From Titration Curve Sorting out some confusing terms. Postequivalence point (v > 25 ml): To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. At the equivalence point, the number. Ph is determined by the amount of excess strong base titrant added; Since both samples are titrated. Equivalence point means the. Determine Equivalence Point From Titration Curve.
From chemistry.stackexchange.com
Titration of CH3COONa with HCl and pKa determination from half equivalence point Chemistry Determine Equivalence Point From Titration Curve Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Ph is determined by the amount of excess strong base titrant added; Postequivalence point (v > 25 ml): \ce{naoh}\) is required to reach the end point when titrated. Equivalence point (v = 25 ml): Suppose that a titration is performed and \(20.70. Determine Equivalence Point From Titration Curve.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Determine Equivalence Point From Titration Curve Suppose that a titration is performed and \(20.70 \: Sorting out some confusing terms. Postequivalence point (v > 25 ml): The equivalence point of a titration. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. A drastic rise in ph is observed as the solution composition transitions from acidic to either. Determine Equivalence Point From Titration Curve.
From mungfali.com
Equivalence Points On Titration Graph Determine Equivalence Point From Titration Curve To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Postequivalence point (v > 25 ml): The equivalence point of a titration. Equivalence point (v = 25 ml): \ce{naoh}\) is required to reach the end point when titrated. A drastic rise in ph is observed as the solution. Determine Equivalence Point From Titration Curve.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Determine Equivalence Point From Titration Curve Suppose that a titration is performed and \(20.70 \: At the equivalence point, the number. The equivalence point of a titration. Equivalence point (v = 25 ml): Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Since both samples are titrated. \ce{naoh}\) is required to reach the end point when titrated.. Determine Equivalence Point From Titration Curve.
From www.youtube.com
How to Calculate the Volume of Titrant Needed to Reach Equivalence Point Titration Curve Example Determine Equivalence Point From Titration Curve \ce{naoh}\) is required to reach the end point when titrated. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Sorting out some confusing terms. Suppose that a titration is performed and \(20.70 \: To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only. Determine Equivalence Point From Titration Curve.
From mungfali.com
Titration Curve Labeled Determine Equivalence Point From Titration Curve A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. At the equivalence point, the number. Postequivalence point (v > 25 ml): Suppose that a titration is performed and \(20.70 \: Equivalence point (v = 25 ml): To evaluate the relationship between a. Determine Equivalence Point From Titration Curve.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Determine Equivalence Point From Titration Curve Postequivalence point (v > 25 ml): Sorting out some confusing terms. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. Equivalence point (v = 25 ml): Since both samples are titrated. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the. Determine Equivalence Point From Titration Curve.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Determine Equivalence Point From Titration Curve To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Since both samples are titrated. Sorting out some confusing terms. Equivalence point (v = 25 ml): Suppose that a titration is performed and \(20.70 \: Equivalence point means the point during titration at which the titrant added has. Determine Equivalence Point From Titration Curve.
From schoolbag.info
Titration and Buffers Acids and Bases Determine Equivalence Point From Titration Curve Suppose that a titration is performed and \(20.70 \: The equivalence point of a titration. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Postequivalence point (v > 25 ml): Since both samples are titrated. A drastic rise in ph is observed as the solution composition transitions from acidic to either. Determine Equivalence Point From Titration Curve.
From srkzhxhdjshcc.blogspot.com
How To Find Half Equivalence Point On Titration Curve Excel Is there any good way to find a Determine Equivalence Point From Titration Curve \ce{naoh}\) is required to reach the end point when titrated. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Since both samples are titrated. Sorting out some confusing terms. Suppose that a titration is performed and \(20.70 \: Postequivalence point (v > 25 ml): Equivalence point (v = 25 ml): The. Determine Equivalence Point From Titration Curve.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Chemistry JoVe Determine Equivalence Point From Titration Curve A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Suppose that a titration is performed and \(20.70 \: Ph is determined by the amount. Determine Equivalence Point From Titration Curve.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point. pH and pKa relation Determine Equivalence Point From Titration Curve Suppose that a titration is performed and \(20.70 \: Equivalence point (v = 25 ml): Postequivalence point (v > 25 ml): \ce{naoh}\) is required to reach the end point when titrated. Ph is determined by the amount of excess strong base titrant added; Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte. Determine Equivalence Point From Titration Curve.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Determine Equivalence Point From Titration Curve At the equivalence point, the number. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. Equivalence point (v = 25 ml): Postequivalence point (v > 25 ml): To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Sorting. Determine Equivalence Point From Titration Curve.
From chemistrytalk.org
Titration Curves & Equivalence Point Calculations ChemTalk Determine Equivalence Point From Titration Curve Sorting out some confusing terms. At the equivalence point, the number. Since both samples are titrated. Postequivalence point (v > 25 ml): Ph is determined by the amount of excess strong base titrant added; Suppose that a titration is performed and \(20.70 \: To evaluate the relationship between a titration’s equivalence point and its end point we need to construct. Determine Equivalence Point From Titration Curve.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators of Acid Base Titration Determine Equivalence Point From Titration Curve Postequivalence point (v > 25 ml): Ph is determined by the amount of excess strong base titrant added; A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. \ce{naoh}\) is required to reach the end point when titrated. Equivalence point means the point. Determine Equivalence Point From Titration Curve.
From goodttorials.blogspot.com
How To Find Equivalence Point From Titration Data Determine Equivalence Point From Titration Curve Sorting out some confusing terms. Postequivalence point (v > 25 ml): Equivalence point (v = 25 ml): Ph is determined by the amount of excess strong base titrant added; \ce{naoh}\) is required to reach the end point when titrated. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid. Determine Equivalence Point From Titration Curve.
From vimeo.com
Determining the Equivalence Point from a Titration Curve on Vimeo Determine Equivalence Point From Titration Curve At the equivalence point, the number. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Postequivalence point (v > 25 ml): Sorting out some confusing terms. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for. Determine Equivalence Point From Titration Curve.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Determine Equivalence Point From Titration Curve Since both samples are titrated. The equivalence point of a titration. Sorting out some confusing terms. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Equivalence point (v = 25 ml): Ph is determined by the amount of excess strong base titrant added; Postequivalence point (v > 25 ml): Suppose that. Determine Equivalence Point From Titration Curve.
From socratic.org
Titration curve? Please help... Socratic Determine Equivalence Point From Titration Curve Postequivalence point (v > 25 ml): To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Suppose that a titration is performed and \(20.70 \: The equivalence point of a titration. \ce{naoh}\) is required to reach the end point when titrated. Since both samples are titrated. At the. Determine Equivalence Point From Titration Curve.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Determine Equivalence Point From Titration Curve \ce{naoh}\) is required to reach the end point when titrated. Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. Since both samples are titrated.. Determine Equivalence Point From Titration Curve.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Determine Equivalence Point From Titration Curve Equivalence point (v = 25 ml): Since both samples are titrated. A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral (for the strong acid sample) or basic (for the weak. Ph is determined by the amount of excess strong base titrant added; Postequivalence point (v > 25 ml): Equivalence point means the. Determine Equivalence Point From Titration Curve.
From philschatz.com
AcidBase Titrations · Chemistry Determine Equivalence Point From Titration Curve Equivalence point means the point during titration at which the titrant added has completely neutralized the analyte solution. Sorting out some confusing terms. At the equivalence point, the number. Ph is determined by the amount of excess strong base titrant added; Equivalence point (v = 25 ml): Since both samples are titrated. Suppose that a titration is performed and \(20.70. Determine Equivalence Point From Titration Curve.
From mungfali.com
Endpoint Titration Curve Determine Equivalence Point From Titration Curve Suppose that a titration is performed and \(20.70 \: To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. At the equivalence point, the number. Equivalence point (v = 25 ml): A drastic rise in ph is observed as the solution composition transitions from acidic to either neutral. Determine Equivalence Point From Titration Curve.
From www.youtube.com
How to Find the Equivalence Point on a Titration Graph In Excel YouTube Determine Equivalence Point From Titration Curve Sorting out some confusing terms. Ph is determined by the amount of excess strong base titrant added; At the equivalence point, the number. Suppose that a titration is performed and \(20.70 \: To evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Equivalence point means the point during. Determine Equivalence Point From Titration Curve.