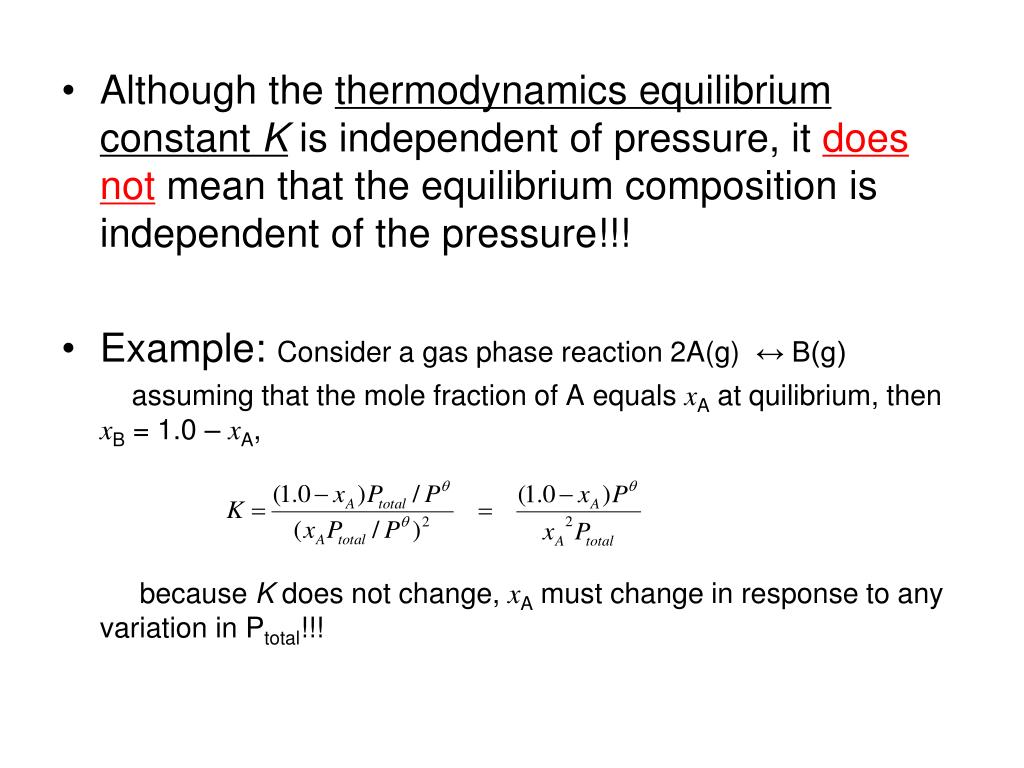
How To Calculate Pressure Equilibrium Constant . with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains. calculation of an equilibrium constant. Substitute into the equilibrium expression. The equilibrium constant for a reaction is calculated from the equilibrium. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. first, calculate the partial pressure for \(\ce{h2o}\) by subtracting the partial pressure of \(\ce{h2}\) from the total. it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. determine all equilibrium concentrations or partial pressures using an ice chart.
from www.slideserve.com
it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. Substitute into the equilibrium expression. The equilibrium constant for a reaction is calculated from the equilibrium. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. determine all equilibrium concentrations or partial pressures using an ice chart. first, calculate the partial pressure for \(\ce{h2o}\) by subtracting the partial pressure of \(\ce{h2}\) from the total. with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how. calculation of an equilibrium constant.
PPT Thermodynamic equilibrium constant K PowerPoint Presentation
How To Calculate Pressure Equilibrium Constant it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. The equilibrium constant for a reaction is calculated from the equilibrium. determine all equilibrium concentrations or partial pressures using an ice chart. it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how. first, calculate the partial pressure for \(\ce{h2o}\) by subtracting the partial pressure of \(\ce{h2}\) from the total. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains. calculation of an equilibrium constant. Substitute into the equilibrium expression.
From www.numerade.com
Expressing equilibrium constant in terms of pressure overview Numerade How To Calculate Pressure Equilibrium Constant with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how. The equilibrium constant for a reaction is calculated from the equilibrium. determine all equilibrium concentrations or partial pressures using an ice chart. Substitute into the equilibrium expression. in this explainer, we will learn how to construct and calculate the. How To Calculate Pressure Equilibrium Constant.
From general.chemistrysteps.com
Kp Equilibrium Constant and Partial Pressure Chemistry Steps How To Calculate Pressure Equilibrium Constant The equilibrium constant for a reaction is calculated from the equilibrium. first, calculate the partial pressure for \(\ce{h2o}\) by subtracting the partial pressure of \(\ce{h2}\) from the total. determine all equilibrium concentrations or partial pressures using an ice chart. with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how.. How To Calculate Pressure Equilibrium Constant.
From www.slideserve.com
PPT Introduction to Chemical Equilibrium PowerPoint Presentation How To Calculate Pressure Equilibrium Constant with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how. it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. determine all equilibrium concentrations or partial pressures using an ice chart. in this explainer, we will. How To Calculate Pressure Equilibrium Constant.
From www.slideserve.com
PPT EQUILIBRIUM CONSTANTS Kp PowerPoint Presentation, free download How To Calculate Pressure Equilibrium Constant determine all equilibrium concentrations or partial pressures using an ice chart. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how. to describe how to calculate equilibrium concentrations from an equilibrium. How To Calculate Pressure Equilibrium Constant.
From learningcampusgale.z21.web.core.windows.net
How To Write An Equilibrium Constant Equation How To Calculate Pressure Equilibrium Constant in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. The equilibrium constant for a reaction is calculated from the equilibrium. it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. first, calculate the partial pressure for \(\ce{h2o}\). How To Calculate Pressure Equilibrium Constant.
From www.slideserve.com
PPT The Equilibrium Constant PowerPoint Presentation, free download How To Calculate Pressure Equilibrium Constant it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how. calculation of an equilibrium constant. to describe how to calculate equilibrium concentrations from an equilibrium constant,. How To Calculate Pressure Equilibrium Constant.
From www.youtube.com
Equilibrium Pressures YouTube How To Calculate Pressure Equilibrium Constant with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. first, calculate the partial pressure for \(\ce{h2o}\) by subtracting the partial pressure of \(\ce{h2}\) from the total. The equilibrium constant for a. How To Calculate Pressure Equilibrium Constant.
From www.tes.com
Physical Chemistry 21 Understanding and Calculating Equilibrium How To Calculate Pressure Equilibrium Constant Substitute into the equilibrium expression. with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that. How To Calculate Pressure Equilibrium Constant.
From study.com
How to Calculate an Equilibrium Constant Kp Using Partial Pressures How To Calculate Pressure Equilibrium Constant in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how. The equilibrium constant for a reaction is calculated from the equilibrium. Substitute into the equilibrium expression. calculation of an equilibrium constant. . How To Calculate Pressure Equilibrium Constant.
From www.slideserve.com
PPT Chapter 14 Chemical Equilibrium PowerPoint Presentation, free How To Calculate Pressure Equilibrium Constant to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains. The equilibrium constant for a reaction is calculated from the equilibrium. it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. Substitute into the equilibrium expression. calculation. How To Calculate Pressure Equilibrium Constant.
From www.numerade.com
Expressing equilibrium constant in terms of pressure overview Numerade How To Calculate Pressure Equilibrium Constant to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains. determine all equilibrium concentrations or partial pressures using an ice chart. calculation of an equilibrium constant. with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how. Substitute into the equilibrium. How To Calculate Pressure Equilibrium Constant.
From printableawusiloxf.z22.web.core.windows.net
Write The Calculation To Work Out Pressure How To Calculate Pressure Equilibrium Constant first, calculate the partial pressure for \(\ce{h2o}\) by subtracting the partial pressure of \(\ce{h2}\) from the total. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains. it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous.. How To Calculate Pressure Equilibrium Constant.
From www.slideserve.com
PPT EQUILIBRIUM CONSTANTS Kp PowerPoint Presentation, free download How To Calculate Pressure Equilibrium Constant to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains. it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. The equilibrium constant for a reaction is calculated from the equilibrium. in this explainer, we will learn. How To Calculate Pressure Equilibrium Constant.
From www.slideserve.com
PPT The Equilibrium Condition, the Equilibrium Constant and How To Calculate Pressure Equilibrium Constant with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how. The equilibrium constant for a reaction is calculated from the equilibrium. it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. to describe how to calculate equilibrium. How To Calculate Pressure Equilibrium Constant.
From www.slideserve.com
PPT The Equilibrium Constant PowerPoint Presentation, free download How To Calculate Pressure Equilibrium Constant Substitute into the equilibrium expression. The equilibrium constant for a reaction is calculated from the equilibrium. it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. first, calculate the partial pressure for \(\ce{h2o}\) by subtracting the partial pressure of \(\ce{h2}\) from the total. to describe. How To Calculate Pressure Equilibrium Constant.
From www.youtube.com
Worked example Calculating the equilibrium total pressure after a How To Calculate Pressure Equilibrium Constant first, calculate the partial pressure for \(\ce{h2o}\) by subtracting the partial pressure of \(\ce{h2}\) from the total. with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. calculation of an equilibrium. How To Calculate Pressure Equilibrium Constant.
From printablecampuscutler.z22.web.core.windows.net
How To Write An Equilibrium Constant Equation How To Calculate Pressure Equilibrium Constant it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. calculation of an equilibrium constant. first, calculate the partial pressure for \(\ce{h2o}\) by subtracting the partial pressure of \(\ce{h2}\) from the total. with this tool, you can calculate the value of an equilibrium constant. How To Calculate Pressure Equilibrium Constant.
From sharedocnow.blogspot.com
Pressure Equilibrium Constant Expression sharedoc How To Calculate Pressure Equilibrium Constant it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures.. How To Calculate Pressure Equilibrium Constant.
From haipernews.com
How To Find Q Equilibrium Constant Haiper How To Calculate Pressure Equilibrium Constant in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. first, calculate the partial pressure for \(\ce{h2o}\) by subtracting the partial pressure of \(\ce{h2}\) from the total. Substitute into the equilibrium expression. with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how.. How To Calculate Pressure Equilibrium Constant.
From www.youtube.com
Equilibria Calculating the equilibrium constant Kp. Qu. 2 of 2 YouTube How To Calculate Pressure Equilibrium Constant Substitute into the equilibrium expression. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains. The equilibrium constant for a reaction is calculated from the equilibrium. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. first, calculate the partial pressure for. How To Calculate Pressure Equilibrium Constant.
From study.com
How to Write Pressure Equilibrium Constant Expressions Chemistry How To Calculate Pressure Equilibrium Constant determine all equilibrium concentrations or partial pressures using an ice chart. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. with this tool, you can calculate. How To Calculate Pressure Equilibrium Constant.
From www.youtube.com
Gibbs Free Energy Equilibrium Constant, Enthalpy & Entropy Equations How To Calculate Pressure Equilibrium Constant with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how. calculation of an equilibrium constant. The equilibrium constant for a reaction is calculated from the equilibrium. it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. . How To Calculate Pressure Equilibrium Constant.
From answerdbjack.z13.web.core.windows.net
How To Determine The Equilibrium Constant How To Calculate Pressure Equilibrium Constant determine all equilibrium concentrations or partial pressures using an ice chart. it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. calculation of an equilibrium constant. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains.. How To Calculate Pressure Equilibrium Constant.
From www.youtube.com
The equilibrium constant Kp YouTube How To Calculate Pressure Equilibrium Constant with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. The equilibrium constant for a reaction is calculated from the equilibrium. calculation of an equilibrium constant. Substitute into the equilibrium expression. . How To Calculate Pressure Equilibrium Constant.
From www.studocu.com
EquilibriumConstantInTermsofPressure ChemicalBiology2 How To Calculate Pressure Equilibrium Constant determine all equilibrium concentrations or partial pressures using an ice chart. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. Substitute into the equilibrium expression. The equilibrium constant for a reaction is calculated from the equilibrium. with this tool, you can calculate the value of an equilibrium constant for. How To Calculate Pressure Equilibrium Constant.
From www.youtube.com
Using equilibrium constants in terms of concentrations and partial How To Calculate Pressure Equilibrium Constant in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. first, calculate the partial pressure for \(\ce{h2o}\) by subtracting the partial pressure of \(\ce{h2}\) from the total. it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. . How To Calculate Pressure Equilibrium Constant.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation ID5819966 How To Calculate Pressure Equilibrium Constant it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. first, calculate the partial pressure for \(\ce{h2o}\) by subtracting the partial pressure of \(\ce{h2}\) from the total. . How To Calculate Pressure Equilibrium Constant.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID How To Calculate Pressure Equilibrium Constant in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. Substitute into the equilibrium expression. calculation of an equilibrium constant. The equilibrium constant for a reaction is calculated from the equilibrium. it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both. How To Calculate Pressure Equilibrium Constant.
From www.slideserve.com
PPT The Equilibrium State PowerPoint Presentation, free download ID How To Calculate Pressure Equilibrium Constant determine all equilibrium concentrations or partial pressures using an ice chart. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. with this tool, you can calculate the value of an equilibrium constant for a reaction while learning how. calculation of an equilibrium constant. The equilibrium constant for a. How To Calculate Pressure Equilibrium Constant.
From www.numerade.com
Expressing equilibrium constant in terms of pressure overview Numerade How To Calculate Pressure Equilibrium Constant determine all equilibrium concentrations or partial pressures using an ice chart. first, calculate the partial pressure for \(\ce{h2o}\) by subtracting the partial pressure of \(\ce{h2}\) from the total. The equilibrium constant for a reaction is calculated from the equilibrium. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. . How To Calculate Pressure Equilibrium Constant.
From www.youtube.com
How an Equilibrium Constant varies with Temperature Thermodynamics How To Calculate Pressure Equilibrium Constant in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. calculation of an equilibrium constant. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains. with this tool, you can calculate the value of an equilibrium constant for a reaction while. How To Calculate Pressure Equilibrium Constant.
From www.slideserve.com
PPT Thermodynamic equilibrium constant K PowerPoint Presentation How To Calculate Pressure Equilibrium Constant determine all equilibrium concentrations or partial pressures using an ice chart. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. to describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains. The equilibrium constant for a reaction is calculated from the equilibrium.. How To Calculate Pressure Equilibrium Constant.
From www.youtube.com
Calculate equilibrium pressure from [ICE Table] 2018 YouTube How To Calculate Pressure Equilibrium Constant determine all equilibrium concentrations or partial pressures using an ice chart. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. Substitute into the equilibrium expression. it covers an explanation of the terms mole fraction and partial pressure, and looks at k p for both homogeneous and heterogeneous. to. How To Calculate Pressure Equilibrium Constant.
From haipernews.com
How To Calculate Equilibrium Constant At Different Temperatures Haiper How To Calculate Pressure Equilibrium Constant first, calculate the partial pressure for \(\ce{h2o}\) by subtracting the partial pressure of \(\ce{h2}\) from the total. determine all equilibrium concentrations or partial pressures using an ice chart. calculation of an equilibrium constant. in this explainer, we will learn how to construct and calculate the equilibrium constant for partial pressures. with this tool, you can. How To Calculate Pressure Equilibrium Constant.
From haipernews.com
How To Calculate The Equilibrium Constant Haiper How To Calculate Pressure Equilibrium Constant Substitute into the equilibrium expression. calculation of an equilibrium constant. first, calculate the partial pressure for \(\ce{h2o}\) by subtracting the partial pressure of \(\ce{h2}\) from the total. determine all equilibrium concentrations or partial pressures using an ice chart. The equilibrium constant for a reaction is calculated from the equilibrium. to describe how to calculate equilibrium concentrations. How To Calculate Pressure Equilibrium Constant.