Magnesium Chloride Solubility . 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium. Water temperature can have a significant effect on the solubility of compounds. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Refer to the chart below to find reference values per gram of. The solubility of magnesium chloride in water is approximately 35.7 g per 100 ml of water at room temperature (25°c). Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. Learn about the solubility, formula, state, and applications of magnesium chloride, a salt with the molecular formula mgcl2. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻).
from www.numerade.com
Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Water temperature can have a significant effect on the solubility of compounds. Refer to the chart below to find reference values per gram of. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. The solubility of magnesium chloride in water is approximately 35.7 g per 100 ml of water at room temperature (25°c). Learn about the solubility, formula, state, and applications of magnesium chloride, a salt with the molecular formula mgcl2.
SOLVEDMagnesium chloride is an important coagulant used in the
Magnesium Chloride Solubility Learn about the solubility, formula, state, and applications of magnesium chloride, a salt with the molecular formula mgcl2. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium. Water temperature can have a significant effect on the solubility of compounds. Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. The solubility of magnesium chloride in water is approximately 35.7 g per 100 ml of water at room temperature (25°c). Refer to the chart below to find reference values per gram of. Learn about the solubility, formula, state, and applications of magnesium chloride, a salt with the molecular formula mgcl2.
From www.numerade.com
SOLVEDMagnesium chloride is an important coagulant used in the Magnesium Chloride Solubility Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). The solubility of magnesium chloride in water is approximately 35.7 g per 100 ml of water at room temperature (25°c). Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. 133 rows in this section we will apply chemical equilibria to the. Magnesium Chloride Solubility.
From www.researchgate.net
The solubility of magnesium chloride hexahydrate under the different Magnesium Chloride Solubility Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. The solubility of magnesium chloride in water is approximately 35.7 g per 100 ml of water at room temperature (25°c).. Magnesium Chloride Solubility.
From www.youtube.com
Solubility of magnesium sulfate in magnesium chloride YouTube Magnesium Chloride Solubility Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. Learn about the solubility, formula, state, and applications of magnesium chloride, a salt with the molecular formula mgcl2. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. The solubility. Magnesium Chloride Solubility.
From yifengtuo.en.made-in-china.com
Solubility Magnesium Chloride Magnesium Chloride Flakes China Mgcl2 Magnesium Chloride Solubility Water temperature can have a significant effect on the solubility of compounds. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Magnesium chloride salts are highly soluble in water and the. Magnesium Chloride Solubility.
From www.nagwa.com
Question Video Using the Water Solubility Rules to Determine Which Magnesium Chloride Solubility Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Refer to the chart below to find reference values per gram of. Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. 133 rows in this section we will apply. Magnesium Chloride Solubility.
From www.researchgate.net
2) gives the solubility of magnesium in water at 25°C as a function of Magnesium Chloride Solubility Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. The solubility of magnesium chloride in water is approximately 35.7 g per 100 ml of water at room temperature (25°c). Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound.. Magnesium Chloride Solubility.
From media.ed.science.psu.edu
Solubility of ionic solids as a function of temperature Magnesium Chloride Solubility Water temperature can have a significant effect on the solubility of compounds. The solubility of magnesium chloride in water is approximately 35.7 g per 100 ml of water at room temperature (25°c). Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Learn about the solubility, formula, state, and applications of magnesium chloride,. Magnesium Chloride Solubility.
From www.slideserve.com
PPT Salts and Solubility PowerPoint Presentation, free download ID Magnesium Chloride Solubility Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Water temperature can have a significant effect on the solubility of compounds. Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine. Magnesium Chloride Solubility.
From www.researchgate.net
Solubility of sodium sulfate and magnesium sulfate [10]. Download Magnesium Chloride Solubility The solubility of magnesium chloride in water is approximately 35.7 g per 100 ml of water at room temperature (25°c). 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium. Refer to the chart below to find reference values per gram of. Magnesium chloride consists of magnesium cations (mg²⁺). Magnesium Chloride Solubility.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Magnesium Chloride Solubility Learn about the solubility, formula, state, and applications of magnesium chloride, a salt with the molecular formula mgcl2. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. The solubility. Magnesium Chloride Solubility.
From www.chegg.com
Solved Spring 2017 Chem 113 1. (a) What Is The Solubility... Magnesium Chloride Solubility Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Learn about the solubility, formula, state, and applications of magnesium chloride, a salt with the molecular formula mgcl2. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium. Magnesium chloride salts are. Magnesium Chloride Solubility.
From mavink.com
Magnesium Chloride Phase Diagram Magnesium Chloride Solubility Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Refer to the chart below to find reference values per gram of. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and. Magnesium Chloride Solubility.
From dokumen.tips
(PDF) REFERENCE SHEET Magnesium · Magnesium chloride 19.68 High Magnesium Chloride Solubility Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. The solubility of magnesium chloride in water is approximately 35.7 g per 100 ml of water at room temperature (25°c). Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). 133 rows in this section we. Magnesium Chloride Solubility.
From www.researchgate.net
The solubility of magnesium chloride hexahydrate under the different Magnesium Chloride Solubility 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea. Magnesium Chloride Solubility.
From blog.iceslicer.com
Chloride Spotlight What is Magnesium Chloride? Magnesium Chloride Solubility 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium. Water temperature can have a significant effect on the solubility of compounds. Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. Refer to the chart. Magnesium Chloride Solubility.
From armsingle10.pythonanywhere.com
Supreme Mgcl2 And Water Equation All Formulas Of Science Class 9 Ncert Magnesium Chloride Solubility Refer to the chart below to find reference values per gram of. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Water temperature can have a significant effect on the solubility. Magnesium Chloride Solubility.
From www.chegg.com
Solved Solubility product of magnesium chloride In this Magnesium Chloride Solubility Water temperature can have a significant effect on the solubility of compounds. Refer to the chart below to find reference values per gram of. Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). The solubility. Magnesium Chloride Solubility.
From www.youtube.com
Write the chemical formula of Magnesium chloride YouTube Magnesium Chloride Solubility Learn about the solubility, formula, state, and applications of magnesium chloride, a salt with the molecular formula mgcl2. Water temperature can have a significant effect on the solubility of compounds. Refer to the chart below to find reference values per gram of. Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted. Magnesium Chloride Solubility.
From www.slideserve.com
PPT Salts and Solubility PowerPoint Presentation, free download ID Magnesium Chloride Solubility Learn about the solubility, formula, state, and applications of magnesium chloride, a salt with the molecular formula mgcl2. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. Refer to. Magnesium Chloride Solubility.
From www.researchgate.net
The solubility of magnesium chloride hexahydrate under the different Magnesium Chloride Solubility Refer to the chart below to find reference values per gram of. Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Learn about the solubility, formula, state, and applications. Magnesium Chloride Solubility.
From www.numerade.com
SOLVEDMagnesium chloride is an important coagulant used in the Magnesium Chloride Solubility Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Water temperature can have a significant effect on the solubility of compounds. Learn about the solubility, formula, state, and applications of magnesium chloride, a salt with. Magnesium Chloride Solubility.
From www.academia.edu
(PDF) The solubility of magnesium chloride and calcium chloride in near Magnesium Chloride Solubility The solubility of magnesium chloride in water is approximately 35.7 g per 100 ml of water at room temperature (25°c). Learn about the solubility, formula, state, and applications of magnesium chloride, a salt with the molecular formula mgcl2. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Magnesium chloride consists of magnesium. Magnesium Chloride Solubility.
From www.elektramagnesium.com.au
Magnesium Science Magnesium Chloride Solubility Water temperature can have a significant effect on the solubility of compounds. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium. The solubility of magnesium chloride in water is approximately 35.7. Magnesium Chloride Solubility.
From www.chegg.com
Solved (6) Regarding magnesium chloride solubility (a) Is Magnesium Chloride Solubility Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium. The solubility of magnesium chloride in water is approximately 35.7 g. Magnesium Chloride Solubility.
From haibinchem.en.made-in-china.com
Magnesium Chloride/Mgcl2 Food Grade Easy to Be Soluble in Water China Magnesium Chloride Solubility Refer to the chart below to find reference values per gram of. Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. The solubility of magnesium chloride in water is. Magnesium Chloride Solubility.
From www.researchgate.net
SOLUBILITY OF Mg(HU) 2 ·8H 2 O PHASE II IN WATER, MAGNESIUM CHLORIDE Magnesium Chloride Solubility Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Refer to the chart below to find reference values per gram of. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). The solubility of magnesium chloride in water is approximately 35.7 g per 100 ml of water at room temperature (25°c).. Magnesium Chloride Solubility.
From www.slideserve.com
PPT Salts and Solubility PowerPoint Presentation, free download ID Magnesium Chloride Solubility Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Refer to the chart below to find reference values per gram of. The solubility of magnesium chloride in water is approximately 35.7 g per 100 ml of water at room temperature (25°c). Learn about the solubility, formula, state, and applications of magnesium chloride,. Magnesium Chloride Solubility.
From www.researchgate.net
The solubility of magnesium chloride hexahydrate of under different Magnesium Chloride Solubility Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. Water temperature can have a significant effect on the solubility of compounds. Refer to the chart below to find reference values per gram of. Learn about the solubility, formula, state, and applications of magnesium chloride, a salt. Magnesium Chloride Solubility.
From techiescientist.com
Is MgCl2 Ionic or Covalent? Techiescientist Magnesium Chloride Solubility Refer to the chart below to find reference values per gram of. Water temperature can have a significant effect on the solubility of compounds. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Learn about the solubility, formula, state, and applications of magnesium chloride, a salt with the molecular formula mgcl2. 133. Magnesium Chloride Solubility.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Magnesium Chloride Solubility The solubility of magnesium chloride in water is approximately 35.7 g per 100 ml of water at room temperature (25°c). Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water.. Magnesium Chloride Solubility.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Magnesium Chloride Solubility The solubility of magnesium chloride in water is approximately 35.7 g per 100 ml of water at room temperature (25°c). Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce. Magnesium Chloride Solubility.
From www.dexform.com
Solubility Rules Chart in Word and Pdf formats Magnesium Chloride Solubility Refer to the chart below to find reference values per gram of. Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. Water temperature can have a significant effect on. Magnesium Chloride Solubility.
From www.chegg.com
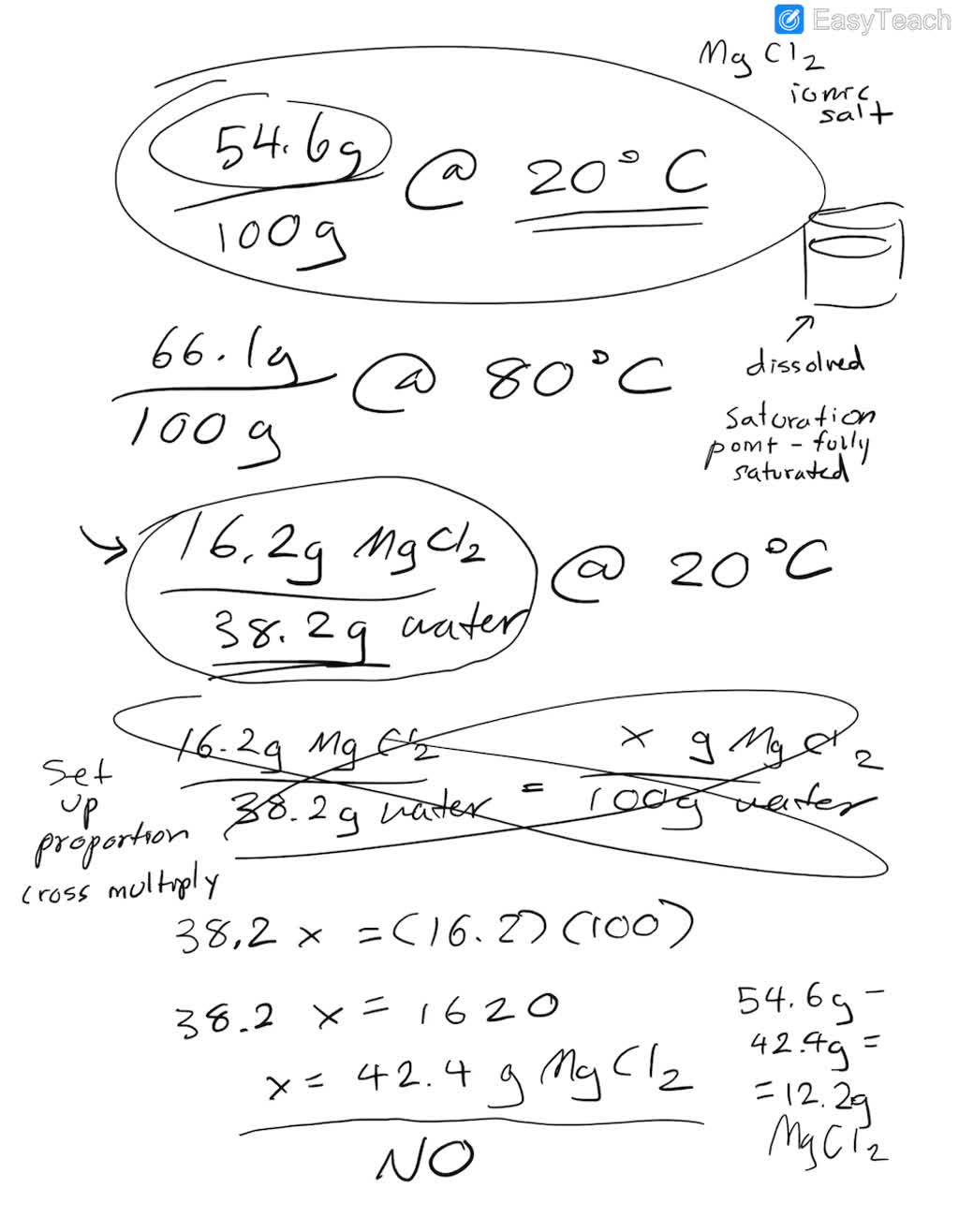
Solved The solubility of magnesium chloride at 20 degree C Magnesium Chloride Solubility Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Refer to the chart below to find reference values per gram of. The solubility of magnesium chloride in water is approximately 35.7 g per 100 ml of water at room temperature (25°c). Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be. Magnesium Chloride Solubility.
From thefitnessmanual.com
Why Is Magnesium Chloride Soluble In Water TheFitnessManual Magnesium Chloride Solubility Refer to the chart below to find reference values per gram of. Magnesium chloride salts are highly soluble in water and the hydrated form of magnesium chloride can be extracted from brine or sea water. 133 rows in this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium. Learn about the chemical. Magnesium Chloride Solubility.
From calebcroomphysci4dummies.weebly.com
Solubility Physical Science For Dummies Magnesium Chloride Solubility The solubility of magnesium chloride in water is approximately 35.7 g per 100 ml of water at room temperature (25°c). Learn about the chemical and physical properties of magnesium chloride (mgcl2), a versatile and important inorganic compound. Water temperature can have a significant effect on the solubility of compounds. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻).. Magnesium Chloride Solubility.