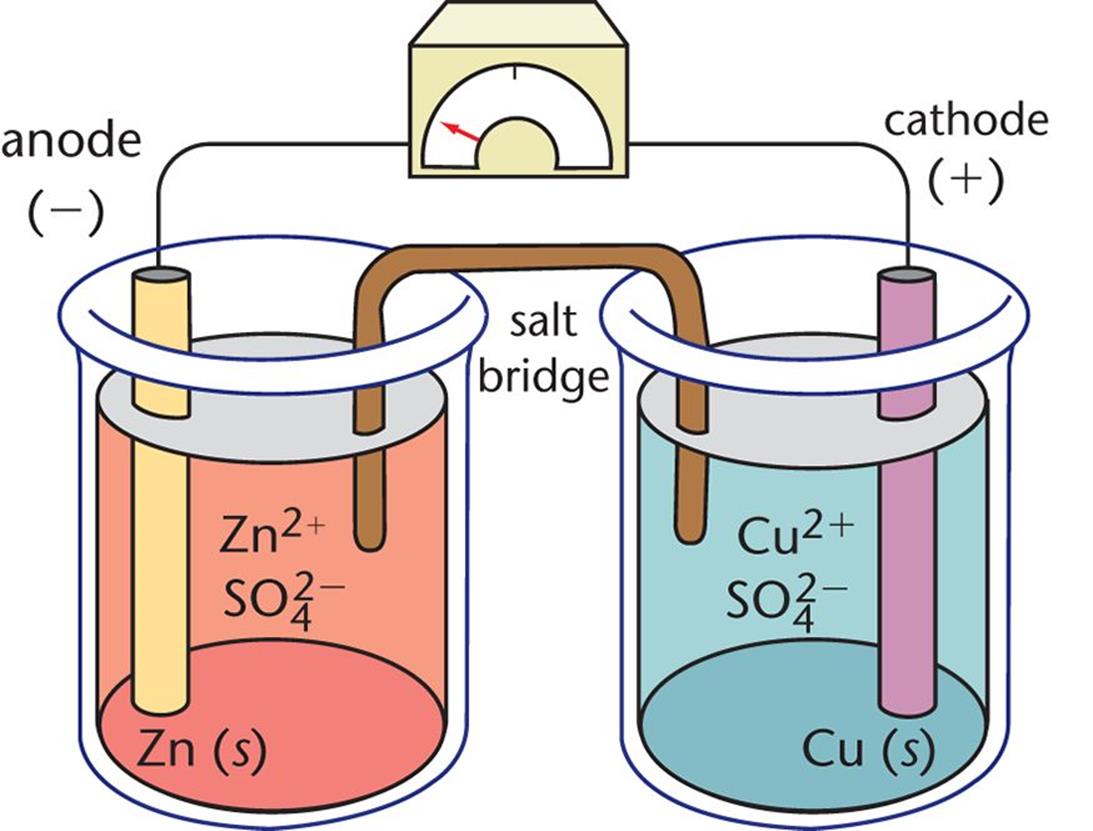
Difference Between Galvanic Cell And Electrochemical . Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Find out the difference between galvanic and electrolytic cells, and how. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical.
from www.myxxgirl.com
Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. Find out the difference between galvanic and electrolytic cells, and how.
Simple Electrochemical Or Galvanic Cell The Daniell Cell Cartoon My
Difference Between Galvanic Cell And Electrochemical Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Find out the difference between galvanic and electrolytic cells, and how. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to.
From www.artofit.org
Electrochemical cell Artofit Difference Between Galvanic Cell And Electrochemical Find out the difference between galvanic and electrolytic cells, and how. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Learn the main difference between electrolytic cells and galvanic. Difference Between Galvanic Cell And Electrochemical.
From www.youtube.com
Comparison of Electrolytic and Galvanic Cells Chy9 Chp7 Difference Between Galvanic Cell And Electrochemical Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. From the above. Difference Between Galvanic Cell And Electrochemical.
From www.alamy.com
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter Difference Between Galvanic Cell And Electrochemical From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Find out the difference between galvanic and electrolytic cells, and how. Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. Learn the basics of redox reactions and. Difference Between Galvanic Cell And Electrochemical.
From www.vrogue.co
What Is The Difference Between Galvanic Cell And Elec vrogue.co Difference Between Galvanic Cell And Electrochemical Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Find out the difference between galvanic and electrolytic cells, and how. From the above differences between galvanic and electrolytic cells, we can conclude. Difference Between Galvanic Cell And Electrochemical.
From exodrddcz.blob.core.windows.net
Difference Between Galvanic And Electrolytic Cell at Brian Chambless blog Difference Between Galvanic Cell And Electrochemical From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic. Difference Between Galvanic Cell And Electrochemical.
From lavelle.chem.ucla.edu
Galvanic vs Voltaic Cells CHEMISTRY COMMUNITY Difference Between Galvanic Cell And Electrochemical Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Find out the difference between galvanic and electrolytic cells, and how. From the above differences between galvanic and electrolytic cells, we can conclude. Difference Between Galvanic Cell And Electrochemical.
From www.alamy.com
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter Difference Between Galvanic Cell And Electrochemical Find out the difference between galvanic and electrolytic cells, and how. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Learn the main difference between electrolytic cells and. Difference Between Galvanic Cell And Electrochemical.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Difference Between Galvanic Cell And Electrochemical Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. Find out the difference between galvanic and electrolytic cells, and how. Learn the main difference between electrolytic cells and galvanic cells, which are. Difference Between Galvanic Cell And Electrochemical.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Difference Between Galvanic Cell And Electrochemical Find out the difference between galvanic and electrolytic cells, and how. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. From the above differences between galvanic and electrolytic cells, we can conclude. Difference Between Galvanic Cell And Electrochemical.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell Difference Between Galvanic Cell And Electrochemical Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to.. Difference Between Galvanic Cell And Electrochemical.
From pediaa.com
Difference Between Electrochemical Cell and Electrolytic Cell Difference Between Galvanic Cell And Electrochemical Find out the difference between galvanic and electrolytic cells, and how. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. From the above differences between galvanic and electrolytic cells, we can conclude. Difference Between Galvanic Cell And Electrochemical.
From www.askiitians.com
Daniell Cell Study Material for IIT JEE askIITians Difference Between Galvanic Cell And Electrochemical Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. Find out the difference between galvanic and electrolytic cells, and how. From the above differences between galvanic and electrolytic cells, we can conclude. Difference Between Galvanic Cell And Electrochemical.
From www.alamy.com
Simple electrochemical or galvanic cell. The Daniell cell Stock Photo Difference Between Galvanic Cell And Electrochemical Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert. Difference Between Galvanic Cell And Electrochemical.
From saylordotorg.github.io
Describing Electrochemical Cells Difference Between Galvanic Cell And Electrochemical Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. Find out the difference between galvanic and electrolytic cells, and how. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. Learn the basics of redox reactions and how to apply them. Difference Between Galvanic Cell And Electrochemical.
From www.pinterest.com
Electrochemistry, featuring electrolysis and fuel cells Difference Between Galvanic Cell And Electrochemical Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. Find out the. Difference Between Galvanic Cell And Electrochemical.
From www.vrogue.co
Grade 12 A Science Galvanic And Electrolytic Cells El vrogue.co Difference Between Galvanic Cell And Electrochemical Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to.. Difference Between Galvanic Cell And Electrochemical.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells Difference Between Galvanic Cell And Electrochemical Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Find out the. Difference Between Galvanic Cell And Electrochemical.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation (Video) JoVE Difference Between Galvanic Cell And Electrochemical Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. Find out the. Difference Between Galvanic Cell And Electrochemical.
From www.vrogue.co
Difference Between Galvanic Cells And Electrolytic Ce vrogue.co Difference Between Galvanic Cell And Electrochemical From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Find out the difference between galvanic and electrolytic cells, and how. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Learn how galvanic cells produce electric current by. Difference Between Galvanic Cell And Electrochemical.
From exodrddcz.blob.core.windows.net
Difference Between Galvanic And Electrolytic Cell at Brian Chambless blog Difference Between Galvanic Cell And Electrochemical Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. Find out the difference between galvanic and electrolytic cells, and how. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Learn the main difference between electrolytic cells and galvanic cells, which are. Difference Between Galvanic Cell And Electrochemical.
From chem.libretexts.org
Electrolytic Cells Chemistry LibreTexts Difference Between Galvanic Cell And Electrochemical Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. Find out the difference between galvanic and electrolytic cells, and how. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Learn the basics of redox reactions and. Difference Between Galvanic Cell And Electrochemical.
From mavink.com
Differentiate Between Electrochemical Cell And Electrolytic Cell Difference Between Galvanic Cell And Electrochemical From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Find out the difference between galvanic and electrolytic cells, and how. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. Learn the basics of redox reactions and how to. Difference Between Galvanic Cell And Electrochemical.
From glossary.periodni.com
Bunsenov članak Chemistry Dictionary & Glossary Difference Between Galvanic Cell And Electrochemical Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Find out the. Difference Between Galvanic Cell And Electrochemical.
From www.pinterest.co.uk
What are the differences between Galvanic cell and Electrolytic cell Difference Between Galvanic Cell And Electrochemical Find out the difference between galvanic and electrolytic cells, and how. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. From the above differences between galvanic and electrolytic cells, we. Difference Between Galvanic Cell And Electrochemical.
From www.myxxgirl.com
Simple Electrochemical Or Galvanic Cell The Daniell Cell Cartoon My Difference Between Galvanic Cell And Electrochemical Find out the difference between galvanic and electrolytic cells, and how. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Learn how galvanic cells produce electric current by. Difference Between Galvanic Cell And Electrochemical.
From www.differencebetween.com
Difference Between Electrochemical Cell and Galvanic Cell Compare the Difference Between Galvanic Cell And Electrochemical Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Find out the difference between galvanic and electrolytic cells, and how. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. From the above differences between galvanic and electrolytic cells, we can conclude. Difference Between Galvanic Cell And Electrochemical.
From mavink.com
Voltaic Cells And Electrolytic Cells Difference Between Galvanic Cell And Electrochemical Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical.. Difference Between Galvanic Cell And Electrochemical.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content Difference Between Galvanic Cell And Electrochemical Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Find out the difference between galvanic and electrolytic cells, and how. Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. Learn how galvanic cells produce electric current by spontaneous redox. Difference Between Galvanic Cell And Electrochemical.
From www.vecteezy.com
Electrochemical cell or Galvanic cell, The Daniell cell 18989226 Vector Difference Between Galvanic Cell And Electrochemical Find out the difference between galvanic and electrolytic cells, and how. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. Learn the main difference between electrolytic cells and galvanic cells, which are both types of electrochemical cells that convert chemical. From the above differences between galvanic and electrolytic cells, we can. Difference Between Galvanic Cell And Electrochemical.
From psiberg.com
Galvanic vs. Electrolytic Cell The Two Types of Electrochemical Cells Difference Between Galvanic Cell And Electrochemical From the above differences between galvanic and electrolytic cells, we can conclude that a galvanic cell produces an electric current with a. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Find out the difference between galvanic and electrolytic cells, and how. Learn how galvanic cells produce electric current by. Difference Between Galvanic Cell And Electrochemical.
From ar.inspiredpencil.com
Galvanic Cell Difference Between Galvanic Cell And Electrochemical Find out the difference between galvanic and electrolytic cells, and how. Learn the basics of redox reactions and how to apply them to electrochemical cells, also known as voltaic cells. Learn how galvanic cells produce electric current by spontaneous redox reactions, while electrolytic cells use external current to. From the above differences between galvanic and electrolytic cells, we can conclude. Difference Between Galvanic Cell And Electrochemical.