Purified Water System Validation As Per Who . This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which allows for. This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. Annex 3, who technical report series, no.1033, 2021 Learn about the three phases of water system validation: This chapter provides information on water quality, processing, and standards for pharmaceutical products and compendial.
from www.alfalaval.us
This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which allows for. This chapter provides information on water quality, processing, and standards for pharmaceutical products and compendial. Annex 3, who technical report series, no.1033, 2021 Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. Learn about the three phases of water system validation: This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water.
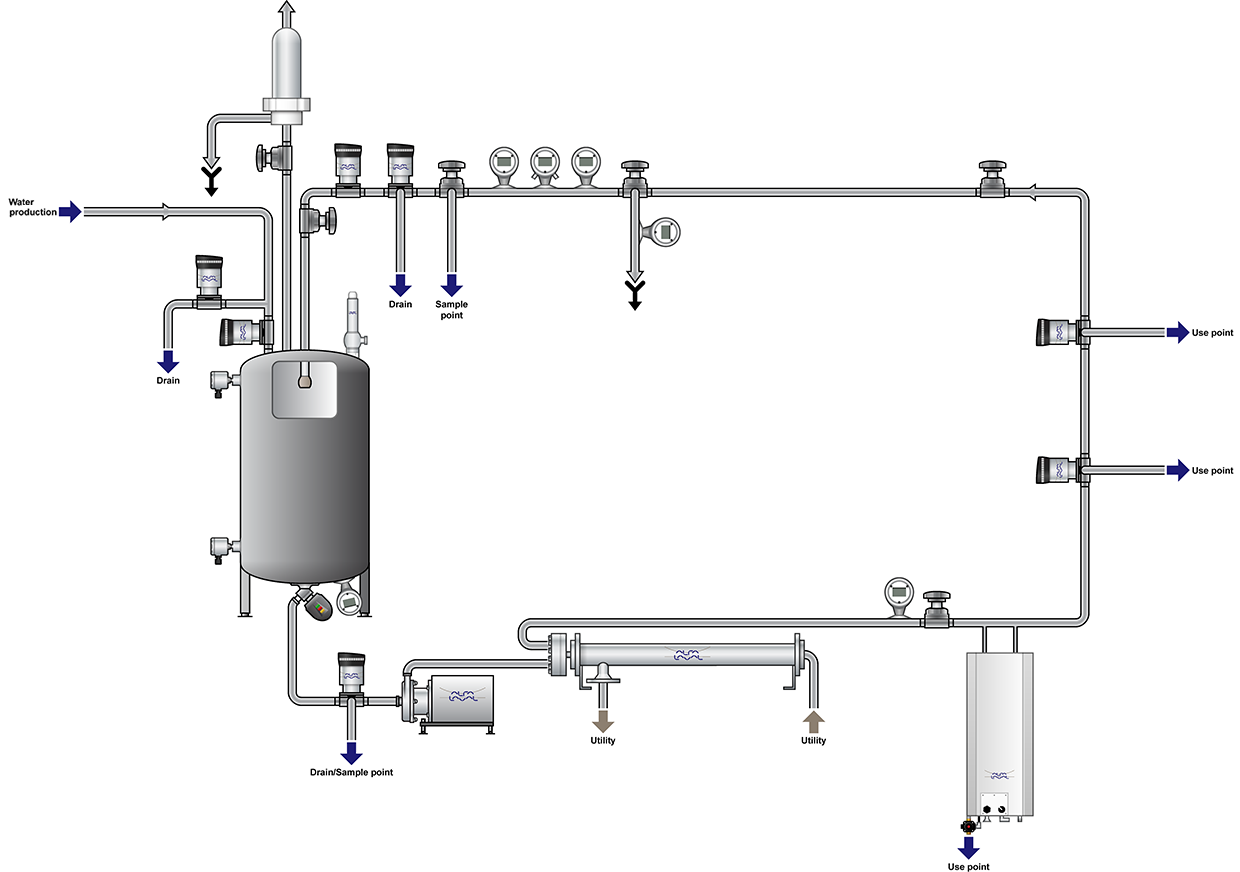
LKH pump and LKH UltraPure Pharmaceutical water systems Alfa Laval
Purified Water System Validation As Per Who This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. Learn about the three phases of water system validation: This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which allows for. This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. This chapter provides information on water quality, processing, and standards for pharmaceutical products and compendial. Annex 3, who technical report series, no.1033, 2021 This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use.
From www.scribd.com
Qualification of Purified Water Systems PDF Verification And Purified Water System Validation As Per Who This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which allows for. This chapter provides information on. Purified Water System Validation As Per Who.
From www.researchgate.net
water system validation life cycle Validation Maintenance Change Purified Water System Validation As Per Who Annex 3, who technical report series, no.1033, 2021 This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which allows for. Learn about the three phases of water system validation: This document provides general principles. Purified Water System Validation As Per Who.
From www.slideserve.com
PPT Pharmaceutical Water Systems PowerPoint Presentation, free Purified Water System Validation As Per Who This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. This chapter provides information on water quality, processing, and standards for pharmaceutical products and compendial. This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. Learn all about water used in pharmaceuticals including requirements for pharmaceutical water. Purified Water System Validation As Per Who.
From www.scribd.com
A Guide To Validating Purified Water PDF Verification And Purified Water System Validation As Per Who This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. This chapter provides information on water quality, processing, and standards for pharmaceutical. Purified Water System Validation As Per Who.
From waterforinjection.com
WHAT IS WATER FOR INJECTION? WFI AND COMPENDIAL WATERS Purified Water System Validation As Per Who This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. Annex 3, who technical report series, no.1033, 2021 This chapter provides information on water quality, processing, and standards for pharmaceutical products and compendial. This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. Learn about the three. Purified Water System Validation As Per Who.
From aqua-chem.com
What are the differences between Purified Water (PW) and Water for Purified Water System Validation As Per Who Learn about the three phases of water system validation: Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. Annex 3, who technical report series, no.1033, 2021 This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. This document is a revision of the who guideline on good manufacturing. Purified Water System Validation As Per Who.
From hxebswbts.blob.core.windows.net
Purified Water System Validation Report at Myrtle Pickel blog Purified Water System Validation As Per Who Learn about the three phases of water system validation: This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. Annex 3, who. Purified Water System Validation As Per Who.
From www.gmp-publishing.com
Qualification of Water Supply Systems GMPVerlag GMP Publishing Purified Water System Validation As Per Who Learn about the three phases of water system validation: This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. This. Purified Water System Validation As Per Who.
From www.youtube.com
VALIDATION OF PURIFIED WATER SYSTEM YouTube Purified Water System Validation As Per Who This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. Annex 3, who technical report series, no.1033, 2021 Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. This chapter provides information on water quality, processing, and standards for pharmaceutical products and compendial. This document provides general principles and. Purified Water System Validation As Per Who.
From www.slideserve.com
PPT Water for Pharmaceutical Use Part 3 Inspection of water Purified Water System Validation As Per Who This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. Learn about the three phases of water system validation: This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. Annex. Purified Water System Validation As Per Who.
From www.totalpharmaceuticaltopics.com
What is Operational Flow of Pharmaceutical Purified water system? Purified Water System Validation As Per Who Learn about the three phases of water system validation: This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. This chapter provides information on water quality, processing, and standards for pharmaceutical products and compendial. Annex 3, who. Purified Water System Validation As Per Who.
From pharmaguidances.com
VALIDATION AND QUALIFICATION Purified Water System Validation As Per Who Annex 3, who technical report series, no.1033, 2021 Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. Learn about the three phases of water system validation: This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which allows for. This document provides guidance on the quality. Purified Water System Validation As Per Who.
From www.honeymanwater.com
Purified Water Systems for Pharmaceutical Manufacturing Purified Water System Validation As Per Who Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. This chapter provides information on water quality, processing, and standards for pharmaceutical. Purified Water System Validation As Per Who.
From www.academia.edu
(PDF) Design, Qualification, and Validation of Water Systems Supriya Purified Water System Validation As Per Who Annex 3, who technical report series, no.1033, 2021 This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which allows for. This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. This chapter provides information on water quality, processing, and standards for pharmaceutical products and. Purified Water System Validation As Per Who.
From www.youtube.com
Purified Water System Validation Water System Qualification Purified Water System Validation As Per Who This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. Annex 3, who technical report series, no.1033, 2021 Learn about the three. Purified Water System Validation As Per Who.
From fyobfaiox.blob.core.windows.net
Purified Water System Validation at Richard Killion blog Purified Water System Validation As Per Who This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. Learn about the three phases of water system validation: This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use,. Purified Water System Validation As Per Who.
From gbu-taganskij.ru
Process Validation An Essential Process PDF PDF, 58 OFF Purified Water System Validation As Per Who This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. Annex 3, who technical report series, no.1033, 2021 This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which allows for. Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water. Purified Water System Validation As Per Who.
From www.researchgate.net
Water system validation life cycle Download Scientific Diagram Purified Water System Validation As Per Who This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. Annex 3, who technical report series, no.1033, 2021 This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. This document is a. Purified Water System Validation As Per Who.
From www.marlo-inc.com
Packaged Purified Water System MARLO Purified Water System Validation As Per Who This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which allows for. This chapter provides information on water quality, processing, and standards for pharmaceutical products and compendial. This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. Learn about the three phases of water system. Purified Water System Validation As Per Who.
From www.slideshare.net
Water system validation by Akshay kakde Purified Water System Validation As Per Who Annex 3, who technical report series, no.1033, 2021 This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which. Purified Water System Validation As Per Who.
From www.edrawmax.com
Water Purified Treatment Process EdrawMax Template Purified Water System Validation As Per Who This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which allows for. This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. Annex 3, who technical report series, no.1033, 2021 This document provides information on different specifications, good practices and validation principles for water for. Purified Water System Validation As Per Who.
From www.honeymanlaboratories.com
Water Testing Labs for Pharmaceutical Manufacture Purified Water System Validation As Per Who This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. Learn about the three phases of water system validation: This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. This document. Purified Water System Validation As Per Who.
From www.scribd.com
Purified Water System Validation PDF Purified Water Verification Purified Water System Validation As Per Who Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. This chapter provides information on water quality, processing, and standards for pharmaceutical products and compendial. This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which allows for. Annex 3, who technical report series, no.1033, 2021 This. Purified Water System Validation As Per Who.
From www.linkedin.com
About pharmaceutical water system validation Purified Water System Validation As Per Who Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. This chapter provides information on water quality, processing, and standards for pharmaceutical products and compendial. This document provides information on different specifications, good practices and validation principles for water. Purified Water System Validation As Per Who.
From www.scribd.com
Validation of Water System Verification And Validation Water Purified Water System Validation As Per Who Annex 3, who technical report series, no.1033, 2021 This chapter provides information on water quality, processing, and standards for pharmaceutical products and compendial. This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. Learn about the three phases of water system validation: This document provides general principles and specific examples of validation and qualification. Purified Water System Validation As Per Who.
From gbu-taganskij.ru
The Q's In Computer System Validation IQ OQ PQ ELeaP, 43 OFF Purified Water System Validation As Per Who This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. Annex 3, who technical report series, no.1033, 2021 This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. This document. Purified Water System Validation As Per Who.
From www.semanticscholar.org
Figure 2 from Qualification of purified water systems Semantic Scholar Purified Water System Validation As Per Who This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which allows for. This document provides guidance on. Purified Water System Validation As Per Who.
From www.scribd.com
Purified Water System Validation 1 PDF Verification And Purified Water System Validation As Per Who Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which allows for. This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. This chapter provides information on water quality,. Purified Water System Validation As Per Who.
From hxebswbts.blob.core.windows.net
Purified Water System Validation Report at Myrtle Pickel blog Purified Water System Validation As Per Who This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which allows for. This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. Learn about the three phases of. Purified Water System Validation As Per Who.
From www.scribd.com
Water System Validation Example Technology Business Purified Water System Validation As Per Who Learn about the three phases of water system validation: Annex 3, who technical report series, no.1033, 2021 This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. This chapter provides information on water quality, processing, and standards for pharmaceutical products and compendial. This document provides information on different specifications, good practices and validation. Purified Water System Validation As Per Who.
From www.seawaterroplants.com
Water purified pharmaceutical water filter toc limits as per usp Purified Water System Validation As Per Who Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. This chapter provides information on water quality, processing, and standards for pharmaceutical products and compendial. This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. Annex 3, who technical report series, no.1033, 2021 This document provides guidance on. Purified Water System Validation As Per Who.
From www.alfalaval.us
LKH pump and LKH UltraPure Pharmaceutical water systems Alfa Laval Purified Water System Validation As Per Who This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. This chapter provides information on water quality, processing, and standards for pharmaceutical products and compendial. Annex 3, who technical report series, no.1033, 2021 Learn about the three phases of water system validation: This document is a revision of the who guideline on good. Purified Water System Validation As Per Who.
From www.total-water.com
How to Get the Ideal Purified Water System for the Pharmaceutical Purified Water System Validation As Per Who Learn about the three phases of water system validation: This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. This document provides information on different specifications, good practices and validation principles for water for pharmaceutical use. Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. Annex 3,. Purified Water System Validation As Per Who.
From desklib.com
Validation of a purified water system PDF Purified Water System Validation As Per Who This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which allows for. This document provides guidance on the quality of water for different pharmaceutical uses, including sterile water. Learn all about water used in pharmaceuticals including requirements for pharmaceutical water system, water quality. This chapter provides information on water quality, processing,. Purified Water System Validation As Per Who.
From pharmawiki.in
Pharmaceutical Water System Ppt Archives Pharmawiki.in Purified Water System Validation As Per Who This document is a revision of the who guideline on good manufacturing practices for water for pharmaceutical use, which allows for. This document provides general principles and specific examples of validation and qualification for pharmaceutical products and processes. This chapter provides information on water quality, processing, and standards for pharmaceutical products and compendial. Learn all about water used in pharmaceuticals. Purified Water System Validation As Per Who.