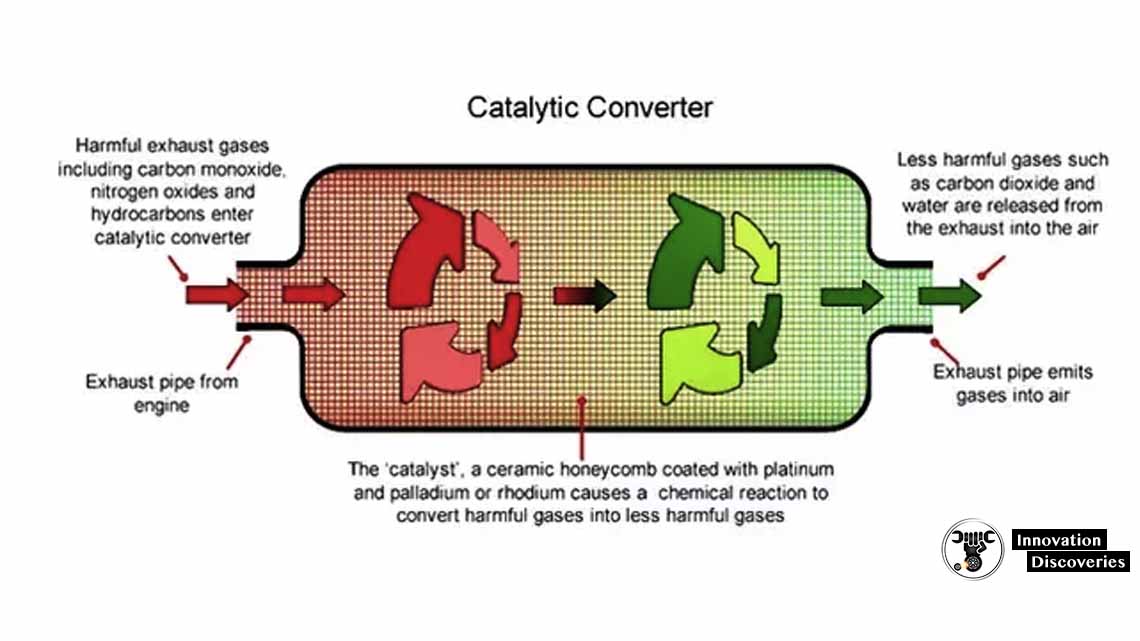
Do Catalytic Converters Remove Sulfur Dioxide . In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be adopted. As this is very stable, there is a loss of catalyst performance. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2 o 4 + so 3 (g) the vanadium(v) oxide is then re. Catalytic convertors in vehicles can be used to remove oxides of nitrogen. A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. The technology also relies on very low sulfur fuel, as sulfur can be stored as sulfate on the storage material. Using fuels which contain low.
from innovationdiscoveries.space
Catalytic convertors in vehicles can be used to remove oxides of nitrogen. As this is very stable, there is a loss of catalyst performance. In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be adopted. The technology also relies on very low sulfur fuel, as sulfur can be stored as sulfate on the storage material. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2 o 4 + so 3 (g) the vanadium(v) oxide is then re. Using fuels which contain low. A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it.
Catalytic Converter (3way)
Do Catalytic Converters Remove Sulfur Dioxide A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. Using fuels which contain low. In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be adopted. The technology also relies on very low sulfur fuel, as sulfur can be stored as sulfate on the storage material. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2 o 4 + so 3 (g) the vanadium(v) oxide is then re. As this is very stable, there is a loss of catalyst performance. A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. Catalytic convertors in vehicles can be used to remove oxides of nitrogen.
From www.carblogindia.com
What Are Catalytic Converters? How Do They Reduce Emissions? » Car Blog India Do Catalytic Converters Remove Sulfur Dioxide Using fuels which contain low. The technology also relies on very low sulfur fuel, as sulfur can be stored as sulfate on the storage material. In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be adopted. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to. Do Catalytic Converters Remove Sulfur Dioxide.
From flasharkracing.com
Catalytic Converter Delete Pipes Pros, Cons, & Considerations Do Catalytic Converters Remove Sulfur Dioxide The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2 o 4 + so 3 (g) the vanadium(v) oxide is then re. As this is very stable, there is a loss of catalyst performance. Catalytic convertors in vehicles can be used to. Do Catalytic Converters Remove Sulfur Dioxide.
From www.eeincorp.com
How Does Catalytic Converters Function? Do Catalytic Converters Remove Sulfur Dioxide The technology also relies on very low sulfur fuel, as sulfur can be stored as sulfate on the storage material. Catalytic convertors in vehicles can be used to remove oxides of nitrogen. In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be adopted. The vanadium(v) oxide catalyst converts sulfur dioxide. Do Catalytic Converters Remove Sulfur Dioxide.
From agmetals.com
Understanding Auto Catalytic Converters Do Catalytic Converters Remove Sulfur Dioxide Catalytic convertors in vehicles can be used to remove oxides of nitrogen. Using fuels which contain low. In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be adopted. A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of. Do Catalytic Converters Remove Sulfur Dioxide.
From www.youtube.com
How 3 way catalytic converters work by Howstuffinmycarworks YouTube Do Catalytic Converters Remove Sulfur Dioxide Using fuels which contain low. The technology also relies on very low sulfur fuel, as sulfur can be stored as sulfate on the storage material. As this is very stable, there is a loss of catalyst performance. In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be adopted. The vanadium(v). Do Catalytic Converters Remove Sulfur Dioxide.
From automotivesblog.com
Features of Catalytic Converters and What They Can be Replaced with AUTOMOTIVESBLOG Do Catalytic Converters Remove Sulfur Dioxide A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be adopted. The technology also relies on very low sulfur fuel, as sulfur can be stored as sulfate. Do Catalytic Converters Remove Sulfur Dioxide.
From www.youtube.com
How To Remove Your Catalytic Converter YouTube Do Catalytic Converters Remove Sulfur Dioxide Using fuels which contain low. Catalytic convertors in vehicles can be used to remove oxides of nitrogen. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2 o 4 + so 3 (g) the vanadium(v) oxide is then re. A catalytic converter. Do Catalytic Converters Remove Sulfur Dioxide.
From www.youtube.com
Maximizing sulfur dioxide removal in flue gases using zirconia oxygen analyzers YouTube Do Catalytic Converters Remove Sulfur Dioxide Catalytic convertors in vehicles can be used to remove oxides of nitrogen. The technology also relies on very low sulfur fuel, as sulfur can be stored as sulfate on the storage material. As this is very stable, there is a loss of catalyst performance. In addition to the obvious requirement of lowering of sulphur content in the fuel, there are. Do Catalytic Converters Remove Sulfur Dioxide.
From pubs.acs.org
TwoPhase Dynamic Modeling and Simulation of Transport and Reaction in Catalytic Sulfur Dioxide Do Catalytic Converters Remove Sulfur Dioxide A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2 o 4 + so 3 (g) the vanadium(v) oxide. Do Catalytic Converters Remove Sulfur Dioxide.
From enggcyclopedia.com
Sulphur Recovery Unit EnggCyclopedia Do Catalytic Converters Remove Sulfur Dioxide In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be adopted. Catalytic convertors in vehicles can be used to remove oxides of nitrogen. A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. As this is very. Do Catalytic Converters Remove Sulfur Dioxide.
From iscrapapp.com
How Do Catalytic Converters Work? Do Catalytic Converters Remove Sulfur Dioxide A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2 o 4 + so 3 (g) the vanadium(v) oxide. Do Catalytic Converters Remove Sulfur Dioxide.
From azurechemical.com
How Does a Selective Catalytic Reduction System Work? Do Catalytic Converters Remove Sulfur Dioxide A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2 o 4 + so 3 (g) the vanadium(v) oxide. Do Catalytic Converters Remove Sulfur Dioxide.
From innovationdiscoveries.space
Catalytic Converter (3way) Do Catalytic Converters Remove Sulfur Dioxide Catalytic convertors in vehicles can be used to remove oxides of nitrogen. A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. Using fuels which contain low. In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be. Do Catalytic Converters Remove Sulfur Dioxide.
From www.savemyexams.co.uk
Nitrogen Oxides & Sulfur Dioxide (4.2.3) Edexcel IGCSE Chemistry Revision Notes 2019 Save My Do Catalytic Converters Remove Sulfur Dioxide Catalytic convertors in vehicles can be used to remove oxides of nitrogen. A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s). Do Catalytic Converters Remove Sulfur Dioxide.
From ar.inspiredpencil.com
How A Catalytic Converter Works Do Catalytic Converters Remove Sulfur Dioxide A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. The technology also relies on very low sulfur fuel, as sulfur can be stored as sulfate on the storage material. Catalytic convertors in vehicles can be used to remove oxides of nitrogen. In addition to the obvious. Do Catalytic Converters Remove Sulfur Dioxide.
From www.darcarsusedcarcenter.com
Is It Time to Replace Your Catalytic Converter? DARCARS Used Car & Service Center of Frederick Do Catalytic Converters Remove Sulfur Dioxide A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be adopted. Using fuels which contain low. The technology also relies on very low sulfur fuel, as sulfur. Do Catalytic Converters Remove Sulfur Dioxide.
From www.researchgate.net
Figure S1 Schematic illustration of automotive catalytic converter and... Download Scientific Do Catalytic Converters Remove Sulfur Dioxide The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2 o 4 + so 3 (g) the vanadium(v) oxide is then re. A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming. Do Catalytic Converters Remove Sulfur Dioxide.
From low-offset.com
Catalytic Converter Delete Explained Low Offset Do Catalytic Converters Remove Sulfur Dioxide In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be adopted. The technology also relies on very low sulfur fuel, as sulfur can be stored as sulfate on the storage material. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g). Do Catalytic Converters Remove Sulfur Dioxide.
From autotrends.org
The Function and Purpose of a Catalytic Converter Auto Trends Magazine Do Catalytic Converters Remove Sulfur Dioxide A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. As this is very stable, there is a loss of catalyst performance. Catalytic convertors in vehicles can be used to remove oxides of nitrogen. In addition to the obvious requirement of lowering of sulphur content in the. Do Catalytic Converters Remove Sulfur Dioxide.
From www.mdpi.com
Catalysts Free FullText A Comprehensive Review on Catalytic Oxidative Desulfurization of Do Catalytic Converters Remove Sulfur Dioxide Catalytic convertors in vehicles can be used to remove oxides of nitrogen. Using fuels which contain low. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2 o 4 + so 3 (g) the vanadium(v) oxide is then re. A catalytic converter. Do Catalytic Converters Remove Sulfur Dioxide.
From instagrid.me
The Science Behind Catalytic Converters and Their Role in Reducing Harmful Emissions Instagrid.me Do Catalytic Converters Remove Sulfur Dioxide The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2 o 4 + so 3 (g) the vanadium(v) oxide is then re. As this is very stable, there is a loss of catalyst performance. Catalytic convertors in vehicles can be used to. Do Catalytic Converters Remove Sulfur Dioxide.
From www.savemyexams.co.uk
Alternative Fuels (1.9.4) Edexcel International A Level Chemistry Revision Notes 2017 Save Do Catalytic Converters Remove Sulfur Dioxide The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2 o 4 + so 3 (g) the vanadium(v) oxide is then re. In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be. Do Catalytic Converters Remove Sulfur Dioxide.
From www.learncax.com
Cfd Simulation Of Catalytic Converter LearnCAx Do Catalytic Converters Remove Sulfur Dioxide The technology also relies on very low sulfur fuel, as sulfur can be stored as sulfate on the storage material. Catalytic convertors in vehicles can be used to remove oxides of nitrogen. A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. In addition to the obvious. Do Catalytic Converters Remove Sulfur Dioxide.
From circuitdbdeviatory.z19.web.core.windows.net
How To Change The Catalytic Converter Do Catalytic Converters Remove Sulfur Dioxide Catalytic convertors in vehicles can be used to remove oxides of nitrogen. A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. Using fuels which contain low. The technology also relies on very low sulfur fuel, as sulfur can be stored as sulfate on the storage material.. Do Catalytic Converters Remove Sulfur Dioxide.
From engineerine.com
Do All Cars Have Catalytic Converters? Complete Guide Engineerine Do Catalytic Converters Remove Sulfur Dioxide In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be adopted. As this is very stable, there is a loss of catalyst performance. Catalytic convertors in vehicles can be used to remove oxides of nitrogen. A catalytic converter is a large metal box, bolted to the underside of your car,. Do Catalytic Converters Remove Sulfur Dioxide.
From www.mdpi.com
Processes Free FullText Modelling and MultiObjective Optimization of the Sulphur Dioxide Do Catalytic Converters Remove Sulfur Dioxide As this is very stable, there is a loss of catalyst performance. In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be adopted. Using fuels which contain low. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2. Do Catalytic Converters Remove Sulfur Dioxide.
From flsnova.com
Published at Applied Catalysis Structured sulphur trioxide splitting catalytic systems and Do Catalytic Converters Remove Sulfur Dioxide The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2 o 4 + so 3 (g) the vanadium(v) oxide is then re. A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming. Do Catalytic Converters Remove Sulfur Dioxide.
From knowhow.napaonline.com
How A Catalytic Converter Works » NAPA Blog Do Catalytic Converters Remove Sulfur Dioxide Using fuels which contain low. Catalytic convertors in vehicles can be used to remove oxides of nitrogen. The technology also relies on very low sulfur fuel, as sulfur can be stored as sulfate on the storage material. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o. Do Catalytic Converters Remove Sulfur Dioxide.
From low-offset.com
Catalytic Converter Delete Explained Low Offset Do Catalytic Converters Remove Sulfur Dioxide As this is very stable, there is a loss of catalyst performance. The technology also relies on very low sulfur fuel, as sulfur can be stored as sulfate on the storage material. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2. Do Catalytic Converters Remove Sulfur Dioxide.
From diagrampartlacquering.z19.web.core.windows.net
Parts Of A Catalytic Converter Do Catalytic Converters Remove Sulfur Dioxide Using fuels which contain low. In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be adopted. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2 o 4 + so 3 (g). Do Catalytic Converters Remove Sulfur Dioxide.
From ar.inspiredpencil.com
How A Catalytic Converter Works Do Catalytic Converters Remove Sulfur Dioxide The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2 o 4 + so 3 (g) the vanadium(v) oxide is then re. As this is very stable, there is a loss of catalyst performance. The technology also relies on very low sulfur. Do Catalytic Converters Remove Sulfur Dioxide.
From mycuglobal.org
HOW THE CATALYTIC CONVERTER WORKS AND COSTS My CU Global Do Catalytic Converters Remove Sulfur Dioxide A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. As this is very stable, there is a loss of catalyst performance. Catalytic convertors in vehicles can be used to remove oxides of nitrogen. Using fuels which contain low. The vanadium(v) oxide catalyst converts sulfur dioxide into. Do Catalytic Converters Remove Sulfur Dioxide.
From engineeringlearner.com
What is Catalytic Converter? Engineering Learner Do Catalytic Converters Remove Sulfur Dioxide As this is very stable, there is a loss of catalyst performance. In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies that can be adopted. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v. Do Catalytic Converters Remove Sulfur Dioxide.
From www.youtube.com
Easiest Catalytic Converter Cleaning And Inspection !!! YouTube Do Catalytic Converters Remove Sulfur Dioxide A catalytic converter is a large metal box, bolted to the underside of your car, that has two pipes coming out of it. The technology also relies on very low sulfur fuel, as sulfur can be stored as sulfate on the storage material. In addition to the obvious requirement of lowering of sulphur content in the fuel, there are strategies. Do Catalytic Converters Remove Sulfur Dioxide.
From exoldzyqx.blob.core.windows.net
How Do You Remove A Catalytic Converter at Stuart Vaca blog Do Catalytic Converters Remove Sulfur Dioxide Using fuels which contain low. The vanadium(v) oxide catalyst converts sulfur dioxide into sulfur trioxide and is reduced to vanadium(iv) oxide so 2 (g) + v 2 o 5 (s) → v 2 o 4 + so 3 (g) the vanadium(v) oxide is then re. As this is very stable, there is a loss of catalyst performance. Catalytic convertors in. Do Catalytic Converters Remove Sulfur Dioxide.