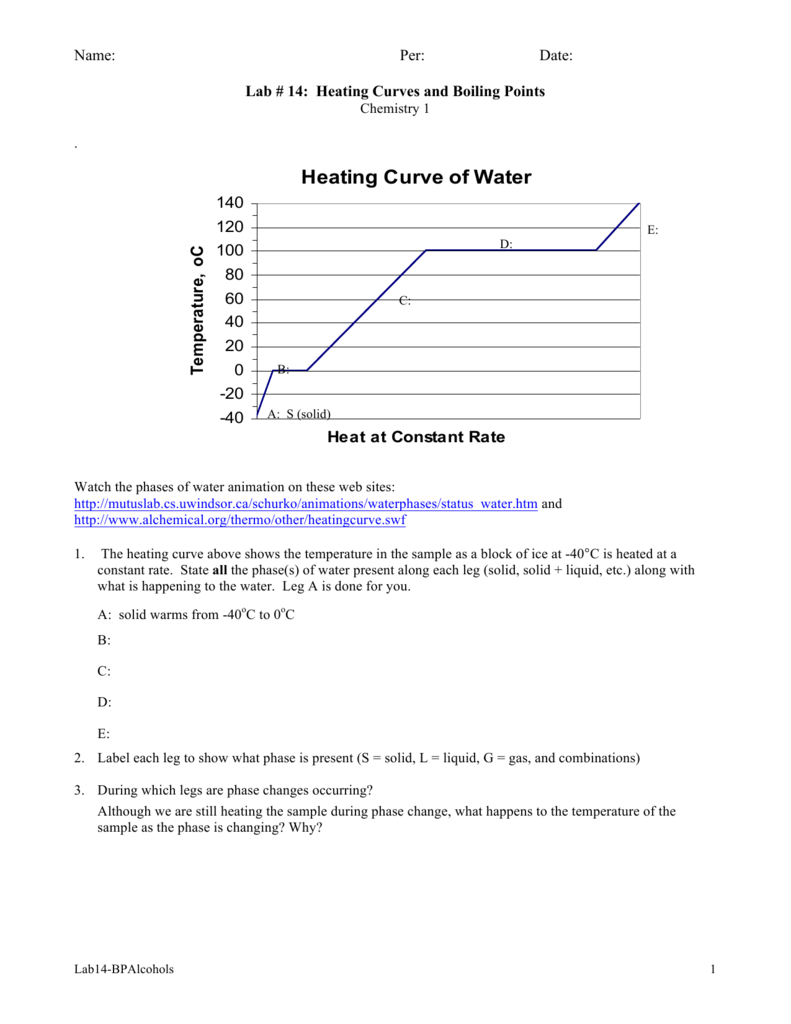
Heating Curve Lab Chemistry . Explore how heat and temperature relate to phase changes. Heating curves show how the temperature changes as a substance is heated up. When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. Cooling curves are the opposite. We take advantage of changes between the gas, liquid, and solid states. Use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] They show how the temperature changes as a substance is cooled down. To calculate the energy changes that accompany phase changes. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective.
from studylib.net
When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. Cooling curves are the opposite. [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] They show how the temperature changes as a substance is cooled down. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. To calculate the energy changes that accompany phase changes. Use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. Heating curves show how the temperature changes as a substance is heated up. Explore how heat and temperature relate to phase changes. We take advantage of changes between the gas, liquid, and solid states.
Heating Curve of Water
Heating Curve Lab Chemistry We take advantage of changes between the gas, liquid, and solid states. Heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. They show how the temperature changes as a substance is cooled down. [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] Explore how heat and temperature relate to phase changes. When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. We take advantage of changes between the gas, liquid, and solid states. To calculate the energy changes that accompany phase changes. Use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes.
From www.worldwisetutoring.com
Heating and Cooling Curves Heating Curve Lab Chemistry [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] Cooling curves are the opposite. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. Explore how heat and temperature relate to phase changes. To calculate the energy changes that accompany phase changes. When a substance being heated or cooled reaches a temperature corresponding to one of its. Heating Curve Lab Chemistry.
From app.jove.com
Heating and Cooling Curves Concept Chemistry JoVe Heating Curve Lab Chemistry We take advantage of changes between the gas, liquid, and solid states. Use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. They show how the temperature changes as a substance is cooled down. Explore how heat and temperature relate to phase changes. [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] Heating curves show how. Heating Curve Lab Chemistry.
From www.slideserve.com
PPT Do Now Hand in specific heat lab & answer the following question on “do now” sheet Heating Curve Lab Chemistry To calculate the energy changes that accompany phase changes. When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. Explore how heat and temperature relate to phase changes. [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] In this simulation, students explore the heating curve for water. Heating Curve Lab Chemistry.
From chem.libretexts.org
8.1 Heating Curves and Phase Changes Chemistry LibreTexts Heating Curve Lab Chemistry They show how the temperature changes as a substance is cooled down. [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] Explore how heat and temperature relate to phase changes. We take advantage of changes between the gas, liquid, and solid states. To calculate the energy changes that accompany phase changes. Heating curves show how the temperature changes as a substance is heated. Heating Curve Lab Chemistry.
From www.showme.com
Heating Curves Science, Chemistry ShowMe Heating Curve Lab Chemistry Explore how heat and temperature relate to phase changes. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. To calculate the energy changes that accompany phase changes. Heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. We take advantage of changes between the gas,. Heating Curve Lab Chemistry.
From studylib.net
Lab Heating Curve Heating Curve Lab Chemistry They show how the temperature changes as a substance is cooled down. When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. Cooling curves are the opposite. Heating curves show how the temperature changes as a substance is heated up. To calculate the energy. Heating Curve Lab Chemistry.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Heating Curve Lab Chemistry When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] Explore how heat and temperature relate to phase changes. Use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. In this. Heating Curve Lab Chemistry.
From chem.libretexts.org
8.1 Heating Curves and Phase Changes Chemistry LibreTexts Heating Curve Lab Chemistry Use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. Heating curves show how the temperature changes as a substance is heated up. Explore how heat and temperature relate to phase changes. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. We take advantage of changes. Heating Curve Lab Chemistry.
From www.youtube.com
How to Read a Heating Curve YouTube Heating Curve Lab Chemistry To calculate the energy changes that accompany phase changes. They show how the temperature changes as a substance is cooled down. When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. Use the heating curve of water simulation to introduce the concepts of heat. Heating Curve Lab Chemistry.
From studylib.net
Lab Heating Curve of Water Heating Curve Lab Chemistry [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] Explore how heat and temperature relate to phase changes. When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. They show how the temperature changes as a substance is cooled down. Heating curves show how the temperature changes. Heating Curve Lab Chemistry.
From schoolbag.info
Figure 7.7. Heating Curve for a Single Compound Heating Curve Lab Chemistry They show how the temperature changes as a substance is cooled down. Use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. Explore how heat and temperature relate to phase changes. Heating curves show how the temperature changes as a substance is heated up. In this simulation, students explore the heating curve for. Heating Curve Lab Chemistry.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Energy YouTube Heating Curve Lab Chemistry Explore how heat and temperature relate to phase changes. To calculate the energy changes that accompany phase changes. Cooling curves are the opposite. When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. In this simulation, students explore the heating curve for water from. Heating Curve Lab Chemistry.
From plot.ly
Creation of a Heating Curve Lab scatter chart made by Asaletsky plotly Heating Curve Lab Chemistry Heating curves show how the temperature changes as a substance is heated up. To calculate the energy changes that accompany phase changes. When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. Cooling curves are the opposite. In this simulation, students explore the heating. Heating Curve Lab Chemistry.
From studylib.net
Heating Curve of Water Heating Curve Lab Chemistry [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] Explore how heat and temperature relate to phase changes. Heating curves show how the temperature changes as a substance is heated up. We take advantage of changes between the gas, liquid, and solid states. They show how the temperature changes as a substance is cooled down. Use the heating curve of water simulation to. Heating Curve Lab Chemistry.
From studylib.net
A.2 Heat Curves Phase diagram Worksheet Key Heating Curve Lab Chemistry Cooling curves are the opposite. We take advantage of changes between the gas, liquid, and solid states. [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. Explore how heat and temperature relate to phase changes. Heating curves. Heating Curve Lab Chemistry.
From spmchemistry.blog.onlinetuition.com.my
3 States of Matter SPM Chemistry Heating Curve Lab Chemistry We take advantage of changes between the gas, liquid, and solid states. Cooling curves are the opposite. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. To calculate the energy changes that accompany phase changes. Heating curves show how the temperature changes as a substance is heated up. They show how the temperature. Heating Curve Lab Chemistry.
From www.smartexamresources.com
IGCSE Chemistry Notes Solids, Liquids And Gases Smart Exam Resources Heating Curve Lab Chemistry Heating curves show how the temperature changes as a substance is heated up. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. Cooling curves are the opposite. To calculate the energy changes that accompany phase changes. They show how the temperature changes as a substance is cooled down. When a substance being heated. Heating Curve Lab Chemistry.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV ] Chemistry YouTube Heating Curve Lab Chemistry We take advantage of changes between the gas, liquid, and solid states. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. Use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. Heating curves show how the temperature changes as a substance is heated up. When a. Heating Curve Lab Chemistry.
From www.youtube.com
Heating Curve of Water Lab Time Lapse YouTube Heating Curve Lab Chemistry Explore how heat and temperature relate to phase changes. When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. To calculate the energy changes that accompany phase changes. They show how the temperature changes as a substance is cooled down. Heating curves show how. Heating Curve Lab Chemistry.
From wisc.pb.unizin.org
Heating Curves and Phase Diagrams (M11Q2) UWMadison Chemistry 103/104 Resource Book Heating Curve Lab Chemistry When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. Cooling curves are the opposite. Explore how heat and temperature relate to phase changes. They show how the temperature changes as a substance is cooled down. [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] Use the. Heating Curve Lab Chemistry.
From schematicdiagramglocer.z19.web.core.windows.net
Heating Curve Chemistry Diagram Heating Curve Lab Chemistry Use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. Explore how heat and temperature relate to phase changes. They show how the temperature changes as a substance is cooled down. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. Cooling curves are the opposite. To. Heating Curve Lab Chemistry.
From quizzmediagerste.z13.web.core.windows.net
Heating Curves Worksheets Heating Curve Lab Chemistry When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. To calculate the energy changes that accompany phase changes. Heating curves show how the temperature changes as a substance is heated up. Explore how heat and temperature relate to phase changes. We take advantage. Heating Curve Lab Chemistry.
From bceweb.org
Heating Curve Chart A Visual Reference of Charts Chart Master Heating Curve Lab Chemistry We take advantage of changes between the gas, liquid, and solid states. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ]. Heating Curve Lab Chemistry.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Heating Curve Lab Chemistry We take advantage of changes between the gas, liquid, and solid states. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. To calculate the energy changes that accompany phase changes. Use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. They show how the temperature changes. Heating Curve Lab Chemistry.
From courses.lumenlearning.com
Phase Transitions Chemistry Heating Curve Lab Chemistry Cooling curves are the opposite. Explore how heat and temperature relate to phase changes. Use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. They show how the temperature changes as a substance is cooled down. We take advantage of changes between the gas, liquid, and solid states. To calculate the energy changes. Heating Curve Lab Chemistry.
From ch301.cm.utexas.edu
heating curve Heating Curve Lab Chemistry [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. Explore how heat and temperature relate to phase changes. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. Cooling curves are. Heating Curve Lab Chemistry.
From www.youtube.com
Lab Phase Changes and Heating Curve YouTube Heating Curve Lab Chemistry They show how the temperature changes as a substance is cooled down. Heating curves show how the temperature changes as a substance is heated up. We take advantage of changes between the gas, liquid, and solid states. [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] Explore how heat and temperature relate to phase changes. In this simulation, students explore the heating curve. Heating Curve Lab Chemistry.
From studylib.net
Heating Curve Lab Heating Curve Lab Chemistry [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] Use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. They show how the temperature changes as a substance is cooled down. When a substance being heated or cooled reaches a. Heating Curve Lab Chemistry.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating Curve Lab Chemistry Explore how heat and temperature relate to phase changes. When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. We take advantage of changes between the gas, liquid, and solid states. Heating curves show how the temperature changes as a substance is heated up.. Heating Curve Lab Chemistry.
From www.youtube.com
Chemistry Heating Curve YouTube Heating Curve Lab Chemistry When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. Explore how heat and temperature relate to phase changes. [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] They show how the temperature changes as a substance is cooled down. Use the heating curve of water simulation. Heating Curve Lab Chemistry.
From www.chegg.com
Solved Q5 Heating Curve 2 Points Consider the following Heating Curve Lab Chemistry When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. Use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. Cooling curves are the opposite. In this simulation, students explore the heating curve for water from a. Heating Curve Lab Chemistry.
From www.youtube.com
Heating Curve and Cooling Curve of Water Enthalpy of Fusion & Vaporization YouTube Heating Curve Lab Chemistry We take advantage of changes between the gas, liquid, and solid states. Cooling curves are the opposite. Explore how heat and temperature relate to phase changes. To calculate the energy changes that accompany phase changes. In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. Heating curves show how the temperature changes as a. Heating Curve Lab Chemistry.
From socratic.org
What are the 6 phase changes along a heating curve? Socratic Heating Curve Lab Chemistry To calculate the energy changes that accompany phase changes. [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] Explore how heat and temperature relate to phase changes. Use the heating curve of water simulation to introduce the concepts of heat capacity and phase changes. Cooling curves are the opposite. Heating curves show how the temperature changes as a substance is heated up. When. Heating Curve Lab Chemistry.
From spmchemistry.blog.onlinetuition.com.my
Cooling Curve SPM Chemistry Heating Curve Lab Chemistry In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] We take advantage of changes between the gas, liquid, and solid states. Cooling curves are the opposite. To calculate the energy changes that accompany phase changes. When a substance being heated or cooled reaches a temperature corresponding to. Heating Curve Lab Chemistry.
From www.chemedx.org
Will a Watched Pot Ever Boil? A Simple, but Effective, Chemistry Lab Constructing a Heating Heating Curve Lab Chemistry Cooling curves are the opposite. [ article:topic, showtoc:no, license:ccbyncsa, licenseversion:30 ] When a substance being heated or cooled reaches a temperature corresponding to one of its phase transitions, further gain or loss of heat is a result. We take advantage of changes between the gas, liquid, and solid states. Use the heating curve of water simulation to introduce the concepts. Heating Curve Lab Chemistry.