Voltmeter Chemistry . Record the electrode potential of the [zn(s) | zn 2+ (aq)] and. The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field. The voltmeter at the very top in the gold color is what measures the cell voltage, or the amount of energy being produced by the electrodes. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is \(e°_{cell}\) = 0.76 v. Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. This reading from the voltmeter is called the. This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different electrodes.
from www.doubtnut.com
This reading from the voltmeter is called the. This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is \(e°_{cell}\) = 0.76 v. The voltmeter at the very top in the gold color is what measures the cell voltage, or the amount of energy being produced by the electrodes. The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field. Record the electrode potential of the [zn(s) | zn 2+ (aq)] and. In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different electrodes. Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips.
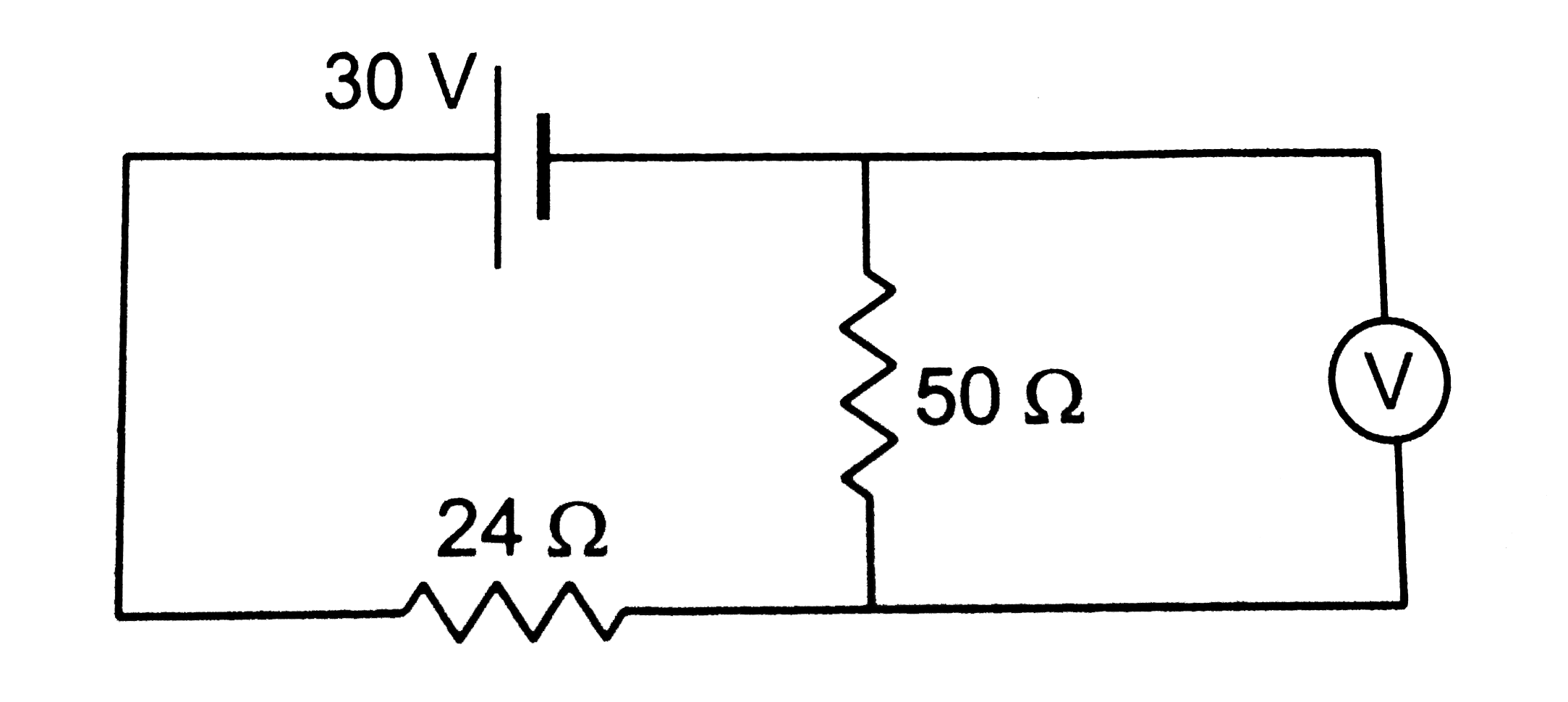
The voltmeter shown in figure reads 18V across the 50(Omega)resistor.
Voltmeter Chemistry This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. Record the electrode potential of the [zn(s) | zn 2+ (aq)] and. The voltmeter at the very top in the gold color is what measures the cell voltage, or the amount of energy being produced by the electrodes. In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different electrodes. Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. This reading from the voltmeter is called the. The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is \(e°_{cell}\) = 0.76 v. This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or.
From www.nagwa.com
Lesson Video Design of the Voltmeter Nagwa Voltmeter Chemistry In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different electrodes. This reading from the voltmeter is called the. Record the electrode potential of the [zn(s) | zn 2+ (aq)] and. The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field. The. Voltmeter Chemistry.
From www.dreamstime.com
Voltmeter stock vector. Illustration of device, isolated 128572412 Voltmeter Chemistry This reading from the voltmeter is called the. Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different electrodes. The. Voltmeter Chemistry.
From www.shutterstock.com
Hoffman Voltmeter Chemical Physical Experiment Stock Vector (Royalty Free) 2395090483 Shutterstock Voltmeter Chemistry In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different electrodes. This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field. The voltmeter shows. Voltmeter Chemistry.
From www.selectschoolsupplies.co.uk
Digital Voltmeter Voltmeter Chemistry This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. This reading from the voltmeter is called the. Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different electrodes. Record. Voltmeter Chemistry.
From www.amazon.in
AC Pointer Voltmeter Voltage Test Meters Good stability 0500V Scale Range Analog Voltmeter Voltmeter Chemistry The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is \(e°_{cell}\) = 0.76 v. In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different electrodes. The voltmeter at the very top in the gold color is what measures the cell. Voltmeter Chemistry.
From www.dreamstime.com
Electrochemical Cell or Galvanic Cell, the Daniell Cell with Voltmeter Stock Vector Voltmeter Chemistry In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different electrodes. This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. This reading from the voltmeter is called the. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she. Voltmeter Chemistry.
From www.chegg.com
Solved AP C Week 15 AF CHEMISTRY Voltmeter Zn(s) Ag(s) 1.0 M Voltmeter Chemistry This reading from the voltmeter is called the. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is \(e°_{cell}\) = 0.76 v. The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field. In this activity, students. Voltmeter Chemistry.
From www.3bscientific.com
DC Voltmeter 1002787 PeakTech U11811 Handheld Analog Measuring Instruments 3B Scientific Voltmeter Chemistry The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field. This reading from the voltmeter is called the. This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. The voltmeter at the very top in the gold color is what measures. Voltmeter Chemistry.
From seniorchem.com
Senior Chemistry Extended Experimental Investigations Voltmeter Chemistry The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field. This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. Record the electrode potential of the [zn(s) | zn 2+ (aq)] and. Join the two metal strips with a voltmeter, using. Voltmeter Chemistry.
From www.kelasplc.com
√ Pengertian, Jenis, Fungsi, Dan Cara Kerja Voltmeter Voltmeter Chemistry Record the electrode potential of the [zn(s) | zn 2+ (aq)] and. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is \(e°_{cell}\) = 0.76 v. Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. In this activity, students will use. Voltmeter Chemistry.
From www.physics2chemistry.com
What is Voltmeter and How does a voltmeter work? Voltmeter Chemistry Record the electrode potential of the [zn(s) | zn 2+ (aq)] and. In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different electrodes. The voltmeter at the very top in the gold color is what measures the cell voltage, or the amount of energy being produced by the electrodes. This video will give. Voltmeter Chemistry.
From www.chescientific.com
GLOBE GLOBE Voltmeter Education Equipment Che Scientific Co. (Hong Kong) Ltd. Voltmeter Chemistry Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field. Record the electrode potential of the [zn(s) | zn 2+ (aq)] and. In this activity, students will use a simulation to create a variety. Voltmeter Chemistry.
From www.chegg.com
Solved AP C Week 15 AF CHEMISTRY Voltmeter Zn(s) Ag(s) 1.0 M Voltmeter Chemistry This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. This reading from the voltmeter is called the. In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different electrodes. Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. The. Voltmeter Chemistry.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and reduction.Simple Voltmeter Chemistry The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is \(e°_{cell}\) = 0.76 v. The voltmeter at the very top in the gold color is what measures the cell voltage, or the amount of energy being produced by the electrodes. This video will give instructions on how. Voltmeter Chemistry.
From www.electricity-magnetism.org
How voltmeter works Analog and Digital Electricity Voltmeter Chemistry The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field. Record the electrode potential of the [zn(s) | zn 2+ (aq)] and. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is \(e°_{cell}\) = 0.76 v.. Voltmeter Chemistry.
From ibexsciences.com
DC Voltmeter Teaching Instrument J0408 IBEX SCIENCES Scientific Supplies, Lab Supplies Voltmeter Chemistry Record the electrode potential of the [zn(s) | zn 2+ (aq)] and. The voltmeter at the very top in the gold color is what measures the cell voltage, or the amount of energy being produced by the electrodes. Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. This reading from the voltmeter is called. Voltmeter Chemistry.
From stock.adobe.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and reduction.Simple Voltmeter Chemistry This reading from the voltmeter is called the. The voltmeter at the very top in the gold color is what measures the cell voltage, or the amount of energy being produced by the electrodes. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is \(e°_{cell}\) = 0.76. Voltmeter Chemistry.
From www.doubtnut.com
The voltmeter shown in figure reads 18V across the 50(Omega)resistor. Voltmeter Chemistry The voltmeter at the very top in the gold color is what measures the cell voltage, or the amount of energy being produced by the electrodes. This reading from the voltmeter is called the. Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. The potential of a cell, measured in volts, is the energy. Voltmeter Chemistry.
From imgbin.com
Hofmann Voltameter Electrolysis Voltmeter PNG, Clipart, Angle, Area, Black And White, Chemistry Voltmeter Chemistry In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different electrodes. This reading from the voltmeter is called the. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is \(e°_{cell}\) = 0.76 v. Join the two metal strips with a. Voltmeter Chemistry.
From edulab.com
Voltmeter, Dual Range Edulab Voltmeter Chemistry In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different electrodes. This reading from the voltmeter is called the. The voltmeter at the very top in the gold color is what measures the cell voltage, or the amount of energy being produced by the electrodes. This video will give instructions on how to. Voltmeter Chemistry.
From courses.lumenlearning.com
Applications of Redox Reactions Voltaic Cells Introductory Chemistry Voltmeter Chemistry The voltmeter at the very top in the gold color is what measures the cell voltage, or the amount of energy being produced by the electrodes. The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field. This reading from the voltmeter is called the. Record the electrode potential of. Voltmeter Chemistry.
From 2012books.lardbucket.org
Standard Potentials Voltmeter Chemistry Record the electrode potential of the [zn(s) | zn 2+ (aq)] and. The voltmeter at the very top in the gold color is what measures the cell voltage, or the amount of energy being produced by the electrodes. This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. In this activity, students. Voltmeter Chemistry.
From www.istockphoto.com
Voltmeter Stock Photo Download Image Now 2015, Battery, Chemistry iStock Voltmeter Chemistry Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field. Record the electrode potential of the [zn(s) | zn 2+ (aq)] and. This reading from the voltmeter is called the. The voltmeter shows that. Voltmeter Chemistry.
From www.electricity-magnetism.org
Digital Voltmeter DVM Description & Characteristics Voltmeter Chemistry This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. The voltmeter at the very top in the gold color is what measures the cell voltage, or the amount of energy being produced by the electrodes. In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with. Voltmeter Chemistry.
From askfilo.com
In the figure shown, voltmeter and ammeter are ideal. Readings of voltmet.. Voltmeter Chemistry This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. This reading from the voltmeter is called the. In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different electrodes. Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. Record. Voltmeter Chemistry.
From gootutorials.blogspot.com
How To Use A Voltmeter To Measure Voltage In A Circuit Voltmeter Chemistry Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. Record the electrode potential of the [zn(s) | zn 2+ (aq)] and. The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field. In this activity, students will use a simulation to create a variety. Voltmeter Chemistry.
From www.surplussales.com
Test Equipment Volt Meters, Leads & Probes Voltmeter Chemistry Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is \(e°_{cell}\) = 0.76 v. Record the electrode potential of the [zn(s) | zn 2+ (aq)] and. The potential of a cell, measured. Voltmeter Chemistry.
From www.shutterstock.com
Voltaic Galvanic Cell Copper Cathode Magnesium Stock Illustration 602037776 Shutterstock Voltmeter Chemistry This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is \(e°_{cell}\) = 0.76 v. The potential of a cell, measured in volts, is the energy needed to move a. Voltmeter Chemistry.
From sciencing.com
How to Use a Voltmeter on a 12 Volt Sciencing Voltmeter Chemistry The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is \(e°_{cell}\) = 0.76 v. The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field. This video will give instructions on how to construct and use a. Voltmeter Chemistry.
From www.schoolspecialty.com
Frey Scientific Economy DC Voltmeter Single Range, 010V (0.2V) Voltmeter Chemistry This reading from the voltmeter is called the. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is \(e°_{cell}\) = 0.76 v. Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. In this activity, students will use a simulation to create. Voltmeter Chemistry.
From www.slideserve.com
PPT AP Chemistry PowerPoint Presentation, free download ID9181174 Voltmeter Chemistry Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is \(e°_{cell}\) = 0.76 v. In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different electrodes.. Voltmeter Chemistry.
From www.shivsons.com
Hoffman Voltmeter, Complete Set Scientific Lab Equipment Manufacturer and Supplier Voltmeter Chemistry Record the electrode potential of the [zn(s) | zn 2+ (aq)] and. The voltmeter at the very top in the gold color is what measures the cell voltage, or the amount of energy being produced by the electrodes. This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. The potential of a. Voltmeter Chemistry.
From www.allaboutcircuits.com
Intro Lab How to Use a Voltmeter to Measure Voltage Basic Projects and Test Equipment Voltmeter Chemistry The voltmeter at the very top in the gold color is what measures the cell voltage, or the amount of energy being produced by the electrodes. Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn. Voltmeter Chemistry.
From www.labkafe.com
Voltmeters ‒ definition, working principle, types Labkafe Voltmeter Chemistry In this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different electrodes. This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field. The voltmeter shows. Voltmeter Chemistry.
From www.classmate4u.com
Digital Voltmeter How it works, Types, Advantages, Disadvantages, Voltmeter Chemistry The voltmeter at the very top in the gold color is what measures the cell voltage, or the amount of energy being produced by the electrodes. This video will give instructions on how to construct and use a galvanic cell ( electrochemical cell or. Join the two metal strips with a voltmeter, using the connecting wires and crocodile clips. The. Voltmeter Chemistry.